Lung cancer screening: Examining the issues
ABSTRACTThe goal of screening is to detect disease at a stage when cure or control is possible, thereby decreasing disease-specific deaths in the population. Many studies have attempted to demonstrate that lung cancer screening using chest radiography or computed tomography (CT) identifies patients with lung cancer and reduces cancer-related mortality. Until recently, there was no evidence confirming a reduction in disease-specific mortality with screening. Early cancer screening should result in a gradual population-wide stage shift toward earlier cancer stages over time, but stage shifting was not reported in early lung cancer screening studies. Lead-time, length-time, and overdiagnosis biases may each have an impact on screening studies reporting survival as an outcome. In this past year, the National Lung Screening Trial reported a significant reduction in cancer-related mortality as a result of screening with chest CT imaging. This will shape the direction of future screening programs.
IS LUNG CANCER OVERDIAGNOSED IN SCREENED POPULATIONS?
Although the apparent benefit of lung cancer screening is susceptible to different sources of bias, overdiagnosis has received the greatest attention on the basis of both theoretical concerns and observations from screening studies. Estimates of lung cancer growth suggest that a typical 10-cm tumor, which is usually large enough to be fatal, has progressed through approximately 40 volume doublings during the course of its existence. In contrast, a more survivable—and clinically detectable—1-cm tumor has progressed through approximately 30 volume doublings.16,17 A lung tumor therefore spends most of its existence relatively undetectable. It has been estimated that the median doubling time is approximately 181 days, and that 22% of lung cancers have doubling times more than 465 days.18 The appearance of tumors on CT may suggest the growth rate, with 1 study showing that solid malignant nodules had a mean doubling time of 149 days, compared with 457 days for partial ground-glass–opacity nodules, and 813 days for pure ground-glass nodules.19
These estimates suggest that if a 1-cm tumor with a history of 30 volume doublings continues to grow at a typical rate (ie, a 181-day doubling time), the patient will die of cancer within 5 years. If the tumor is among the 22% of those with a 465-day doubling time, the survival time would be 12.7 years. For malignant pure ground-glass nodules, the projected time to death is 22 years. Individuals with lung cancer are often elderly, long-term cigarette smokers with emphysema or other chronic health problems—many of whom would die of other causes before their lung cancers progressed enough to cause significant health problems.
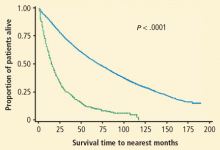
As an argument against the significance of overdiagnosis in lung cancer screening, it has been noted that outcomes are worse for patients identified with early-stage lung cancer in screening studies who do not receive treatment. For example, the results of a study of 1,432 patients with stage I non–small cell lung cancer (NSCLC) are illustrated in Figure 3. Survival was much better in screened patients who were treated than in those who were untreated, with almost all the untreated patients dying within 10 years of diagnosis.20 However, the subjects in this study were atypical of those in most screening studies. Thirty-three percent of the patients had squamous cell carcinoma and 61% had relatively large T2 lesions, compared with a typical screening study comprised of patients with more than 50% T1 lesions and a smaller percentage of squamous cell carcinoma.
Another argument against overdiagnosis comes from gene profiling studies that have compared genetic tumor markers for tumors identified by screening with tumors identified clinically. One study found that the expression profile of 3,231 genes was similar for patients with lung cancer identified by screening or by symptoms.21 However, these investigators also found that nine genes known to be important in tumor growth differed between screened and nonscreened populations.
The significance of overdiagnosis is supported by a long-term follow-up study from the Mayo Clinic chest radiography screening trial, which found that the number of lung cancer cases remained higher in the screening group than the control group (585 vs 500 cases) for up to 28 years after screening, suggesting an overdiagnosis of lung cancer by approximately 85 cases per 500 patients screened (approximately 17%).22 Several studies have also demonstrated that screening populations may have tumors with more favorable histology or clinical characteristics, including higher levels of bronchioloalveolar carcinoma or well-differentiated adenocarcinoma.23–25 Finally, autopsy series have found undiagnosed lung tumors in as many as 1% of patients who died from natural causes, with fewer advanced tumors found in the 1970s than in the 1950s.26,27
These arguments led most to believe randomized controlled trials of CT-based screening were needed. The largest of these, the National Lung Screening Trial (NLST), has recently reported results that will clarify the impact of lung cancer screening on cancer-related mortality.28 This study enrolled 53,456 subjects between the ages of 55 and 74 years with a history of at least 30 pack-years of smoking. Patients were randomized to baseline screening followed by annual screening for 2 years using either low-dose helical CT or chest radiography and outcome follow-up 5 years after randomization. Data analysis after 6 to 8 years of follow-up found 442 lung cancer deaths in the chest radiograph arm versus 354 in the CT arm, representing a 20.3% reduction with CT.29 Screening of 320 patients using low-dose helical CT would be required to avoid each lung cancer death. Thus, after years of debate, it has been demonstrated that it is possible to reduce lung cancer-specific mortality with CT-based screening.
ARE THERE SIGNIFICANT RISKS WITH CT-BASED SCREENING?
Radiation from CT tests is a potential concern, although it is difficult to quantify the importance of this risk. One estimate of CT-related radiation exposure found that annual CT screening of 50% of the eligible population between 50 and 75 years of age in the United States would result in approximately 36,000 new cancers, or a 1.8% increase in the rate of cancer over the expected rate.32 Many patients and health care professionals are already concerned about the degree of radiation exposure from medical diagnostics. A recent study that examined cumulative radiation exposure due to medical imaging in 952,420 adults aged 18 to 64 years found that approximately 57.9% of men and 78.7% of women receive at least some annual health care-related radiation exposure.33 Radiation exposure was considered moderate (> 3–20 mSv/yr) for 18.1% of men and 20.3% of women, and was considered high (> 20–50 mSv/yr) or very high (>50 mSv/hr) for 2.3% of men and 2.1% of women.