Safety issues in vasculitis: Infections and immunizations in the immunosuppressed host
ABSTRACTInfectious diseases are a significant cause of morbidity and mortality in immunosuppressed patients, including those with connective tissue diseases. Both disease and treatment contribute to a predisposition to infection in immunocompromised patients. Significant infection and morbidity occur in 25% to 50% of these patients with a median mortality of 5.2% due to common bacterial infections, such as pneumonia or bacteremia, and opportunistic fungal infections such as Pneumocystis. The lungs, skin, urinary tract, blood, and central nervous system are commonly affected. Pathogens such as Pneumocystis jirovecii, Histoplasma capsulatum, Aspergillus species, herpes zoster, JC virus, Nocardia asteroides, and Nocardia species are increasingly prevalent in immunocompromised patients. Improved recognition, diagnosis, and prevention of these infections are needed to enhance outcomes in these patients.
In 2007, Falagas et al1 provided a systematic review of studies focusing on infection-related morbidity and mortality in patients with connective tissue diseases. Many of the studies reviewed were published prior to the introduction of biologic agents for the treatment of rheumatologic disorders. In 39 studies focusing on infection incidence, patient outcomes, or both in patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis/dermatomyositis, granulomatosis with polyangiitis (GPA, [Wegener’s granulomatosis]), and systemic sclerosis, serious infection developed in 29% of patients and 24% of these died due to the infection with a median attributable mortality of 5.2%. Most of the reported infections were common bacterial syndromes such as pneumonia or bacteremia, and opportunistic fungal (Pneumocystis) infections.
Similarly, in 2006 Alarcón2 reported that 25% to 50% of patients with SLE had significant morbidity primarily from common bacterial infections, with viral, fungal, and parasitic infection less common. Staphylococcus aureus was a common cause of soft tissue infection, septic arthritis, and bacteremia. Streptococcus pneumoniae typically caused respiratory infections, although meningitis and sepsis were reported with SLE. Gram-negative bacteria such as Escherichia coli, Klebsiella species, and Pseudomonas species usually caused urinary tract infections and nosocomial pneumonia. Other bacterial infections included Nocardia species, Mycobacterium tuberculosis, and, rarely, Listeria monocytogenes. The most common viral infection was herpes zoster. Fungal infections included Pneumocystis jirovecii (formerly known as Pneumocystis carinii) and Candida species.
In scleroderma, another connective tissue disease evaluated in the literature by Alarcón,2 reports of bacterial, viral, and fungal infections are limited to case reports. In scleroderma patients, viral infections with cytomegalovirus (CMV), parvovirus B19, and P jirovecii were similar to pathogens observed with SLE.
In polymyositis/dermatomyositis, gram-positive pneumonia affected 15% to 20% of patients and S aureus occurred frequently in the juvenile form of the disease. Herpes zoster was commonly observed, but CMV was relatively rare. Other viral infections included Coxsackie virus, parvovirus B19, and hepatitis C in polymyositis/dermatomyositis. Infection with P jirovecii is frequently fatal in these patients. Other fungal infections seen in polymyositis/dermatomyositis include candidiasis and histoplasmosis.2
Since the approval of antitumor necrosis factor (anti-TNF) agents for RA in the late 1990s, as well as other more recent biologic agents, there has been heightened awareness of infectious complications in rheumatologic patients. A major concern with the anti-TNF agents is the risk of granulomatous infection, particularly mycobacterial disease and dimorphic fungal infections such as histoplasmosis and coccidioidomycosis. Formation of granulomas is the major host defense against mycobacterial infection and is mediated in large part by TNF-alpha. The precise risk of infection associated with each of the various biologic agents is still under study, and rates from randomized trials have differed from postmarketing surveillance studies. Important pathogens associated with biologic agents include Nocardia, CMV, Listeria, Aspergillus, and JC virus (JCV).3,4 Delays in the diagnosis of these infections in immunocompromised patients have led to poor outcomes.
KEY PATHOGENS IN INFECTIONS OF IMMUNOCOMPROMISED HOSTS
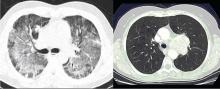
Pneumocystis jirovecii
For many decades, P jirovecii was classified as a protozoan but, based on gene sequencing, the organism has been reclassified as a fungus. P jirovecii is a low-virulence, unicellular organism that is the causative agent of Pneumocystis pneumonia (PCP). Epidemiologically, primary infection most likely occurs in infants and children. Colonization may be transient, entering the airways and then resolving over a period of weeks or months. Alternatively, the organism may enter a latent state similar to tuberculosis with reactivation occurring during times of intense immunosuppression. However, molecular epidemiology studies show that new cases of PCP are likely environmentally acquired through multiple exposures rather than reactivation of latent infection.5,6 Transmission is thought to be airborne from person to person. Pathogenically, the trophic form of the organism attaches to type 1 alveolar cells and remains in the extracellular compartment of the alveoli. This colonization evokes an influx of inflammatory cells (CD8 cells, neutrophils, and macrophages). However, not all colonizations result in pneumonia—even in advanced human immunodeficiency virus (HIV) infection. While there is an innate immunity through alveolar macrophages and pulmonary surfactant, alveolar macrophage response is impaired in HIV when the CD4 count is low. Cell-mediated immunity is the main defense against progression to pneumonia with assistance from costimulatory molecules (such as CD28 and CD2) as well as B cells.
Laboratory diagnosis. P jirovecii cannot be grown in culture for clinical purposes, and it is extremely difficult to culture even in the research setting. Cytologic stains such as the Wright-Giemsa and methamine silver stains are the mainstay of laboratory diagnosis. The yield for P jirovecii from routine expectorated sputum is very low and some laboratories discourage this approach. The sensitivity of nebulized sputum using hypertonic saline ranges from 50% to 90%.9
In patients with acquired immune deficiency syndrome (AIDS), bronchoscopy provides 90% to 98% sensitivity by BAL. Transbronchial biopsy may provide some additional yield over BAL in a few situations, such as patients who have been receiving partial P jirovecii prophylaxis. Immunofluorescence techniques using monoclonal antibodies to P jirovecii are commercially available and are first-line diagnostic tools in some laboratories. Recently, polymerase chain reaction (PCR) assay has been introduced into clinical practice as a reproducible test with high sensitivity.
Primary therapy. Primary therapy for PCP consists of trimethoprim-sulfamethoxazole (TMP-SMX) or pentamidine. TMP-SMX is considered the drug of choice and is usually administered intravenously for 21 days in HIV patients and 14 days for non-HIV patients. The oral form may be used in patients with less severe PCP with a functioning gastrointestinal tract. Common adverse reactions to TMP-SMX include rash, Stevens-Johnson syndrome, neutropenia, changes in pulmonary function, and nausea/vomiting/diarrhea.10 Pentamidine is as effective as TMP-SMX, but is associated with renal toxicity, hypotension, severe hypoglycemia, cardiac arrhythmias, and diabetes.11 It is generally reserved for severe cases of PCP in patients who are allergic to or otherwise intolerant of sulfa. Other treatments include atovaquone and trimethoprim-dapsone. Adjunctive corticosteroids have been shown to be beneficial in moderate to severe PCP in HIV patients to reduce the local host inflammatory response to dead or dying organisms. Recent guidelines have recommended corticosteroids for HIV patients with PCP who have an arterial oxygen pressure of 70 mm Hg or less on room air, or an alveolar-arterial (A-a) gradient of oxygen 35 mm Hg or greater.12 Little is known about the role of adjunctive corticosteroids in non-HIV patients, given a lack of clinical studies.
Prevention. Recent estimates of disease burden from a meta-analysis of 11,900 patients with connective tissue diseases found PCP in 12% of patients with GPA, in 6% of those with polydermatomyositis, in 5% of those with SLE, and in 1% of those with RA.1 Mortality due to PCP is higher in patients with rheumatic diseases, ranging from 30% in RA to 63% in GPA, than in those with HIV (10% to 20%).13 One key risk factor predisposing patients with connective tissue diseases to infection with P jirovecii is recent corticosteroid use. Among patients with connective tissue disease, more than 90% of those infected with P jirovecii have recently received steroid therapy.14 Additionally, in almost all patients with P jirovecii, lymphopenia with absolute lymphocyte counts less than 1,000/mm3 is present.15
In patients with HIV, prophylaxis is initiated at a CD4 level of 200/mm3.13 However, the cutoff is less clear for non-HIV rheumatic patients. A cutoff of less than 300 cells/mm3 has been proposed for prophylaxis of PCP. However, at that range, approximately 50% of patients with connective tissue disease would remain above the threshold.13 One possible solution is to screen by PCR and treat colonization. Other algorithms have been proposed, but there is no general consensus on treatment of non-HIV rheumatic patients.13,16 Generally, prophylaxis should be considered in patients at the highest risk for PCP. These include patients taking prednisone at doses greater than 20 mg/day for 1 month plus a cytotoxic agent, a TNF inhibitor plus glucocorticoids, and methotrexate plus glucocorticoids in GPA.13