Managing the patient with newly diagnosed Parkinson disease
ABSTRACTThe treatment of early Parkinson disease (PD) is generally symptomatic, although therapy that also offers neuroprotection in early-stage PD would be welcomed. Levodopa remains the most effective agent for relief of PD symptoms, but chronic levodopa therapy is associated with motor fluctuations and dyskinesias, and clinicians may therefore opt to postpone its use. Alternatives to levodopa in early PD include monoamine oxidase (MAO)-B inhibitors, amantadine, and dopamine agonists. MAO-B inhibitors have only mild symptomatic effects. Amantadine is associated with improvement in functional disability and, in a subset of PD patients, a robust symptomatic improvement. Dopamine agonists improve symptoms and may have a neuroprotective effect. Partial dopamine agonists, adenosine A2A-receptor antagonists, and safinamide are symptomatic therapies that are under investigation. Neuro protective strategies under study include enhancement of mitochondrial function, antiinflammatory mechanisms, calcium channel blockade, and uric acid elevation. Deep brain stimulation may slow cognitive and motor decline when used in early PD. Stem cell therapy and gene therapy are still under investigation.
SYMPTOMATIC THERAPIES: THE FUTURE
Partial dopamine agonists
Pardoprunox is a partial dopamine agonist with full 5-HT1A–agonist activity. A partial dopamine agonist acts in two ways: (1) It stimulates dopamine production in brain regions with low dopamine tone, and (2) it has dopamine antagonist activity under circumstances of high dopamine sensitivity, theoretically avoiding overstimulation of dopamine receptors. Because it inhibits excessive dopamine effect, pardoprunox may prevent dyskinesia. In addition, because pardoprunox has serotonin agonist activity, it may also act as an antidepressant.
In a phase 2 study, significantly more patients randomized to pardoprunox had a 30% or greater reduction in UPDRS motor score compared with placebo at end-of-dose titration (35.8% for pardoprunox vs 15.7% for placebo; P = .0065) and at end point (50.7% for pardoprunox vs 15.7% for placebo; P < .0001).12
Adenosine A2A-receptor antagonists
Adenosine A2A receptors are located in the basal ganglia, primarily on gamma aminobutyric acid (GABA)–mediated enkephalin-expressing medium spiny neurons in the striatum. These receptors modulate dopamine transmission by opposing D2-receptor activity. The D2 pathway is an indirect pathway that promotes suppression of unnecessary movement.
Two A2A-receptor antagonists have demonstrated efficacy in clinical trials. Vipadenant has been proven effective as monotherapy in phase 2 clinical trials. Preladenant has been shown to improve “off time” as an adjunct to levodopa without increasing dyskinesia.
Safinamide
Safinamide, currently in phase 3 clinical trials, has three mechanisms of action. It is an inhibitor of dopamine reuptake, a reversible inhibitor of MAO-B, and an inhibitor of excessive glutamate release. The addition of safinamide to a stable dose of a single dopamine agonist in patients with early PD resulted in improvement of motor symptoms and cognitive function.13,14
NEUROPROTECTIVE STRATEGIES UNDER INVESTIGATION
Four neuroprotective strategies are under study: enhanced mitochondrial function, antiinflammatory mechanisms, calcium channel blockade, and uric acid elevation.
Enhanced mitochondrial function
Creatine has generated interest as a disease-modifying agent in response to preclinical data showing that it could enhance mitochondrial function and prevent mitochondrial loss in the brain in models of PD. Creatine is now the subject of a large phase 3 National Institutes of Health–sponsored clinical trial in patients with early-stage PD.15
Coenzyme Q10 (CoQ 10) exhibited a trend for neuroprotection at 1,200 mg/d, lowering the total mean UPDRS score compared with placebo in a 16-month study.16 Current efforts are directed at determining whether 1,200 or 2,400 mg/d of CoQ10 are neuroprotective. A nanoparticulate form of CoQ10, 100 mg three times a day, has been shown to produce plasma levels of CoQ10 equivalent to those produced by 1,200-mg doses of the standard form.17 CoQ10 is free of symptomatic effects.
Antiinflammatory mechanisms
Parkinson disease may have an important inflammatory component. A meta-analysis of seven studies showed an overall hazard ratio of 0.85 for development of PD in users of nonaspirin nonsteroidal antiinflammatory drugs (NSAIDs), with each of the seven studies demonstrating a hazard ratio less than 1.18 A similar meta-analysis showed no such association.19 Further study is warranted.
The antidiabetic agent pioglitazone, shown in mice to prevent dopaminergic nigral cell loss, has been entered into a phase 2 clinical trial to assess its antiinflammatory properties in PD.
Calcium channel blockade
A sustained-release formulation of isradipine, an L-type calcium channel blocker, is being studied in a phase 2 clinical trial for the treatment of early PD; experimental evidence in animals suggests that it may be neuroprotective against PD.
Uric acid elevation
Urate concentration in the cerebrospinal fluid predicts progression of PD, with higher levels associated with slower progression of disease.20 Urate may delay oxidative destruction of dopaminergic neurons that occurs with progression of PD. Pharmacologic elevation of uric acid is being explored as a treatment option in PD.
ELECTRODES, VECTORS, AND STEM CELLS
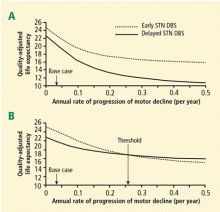
Deep brain stimulation
Stem cell therapy
Stem cells obtained from blastocytes, fibroblasts, bone marrow, or the adult, embryonic, or fetal central nervous system through “molecular alchemy” can form dopaminergic neuroblasts. Given the high cost and potential risks of stem cell therapy, it must be proven superior to DBS to be considered an option for early PD. Several practical problems act as hurdles to successful stem cell therapy. Efficient generation of dopamine-producing neurons and successful grafting are required. Tumor growth is a risk. Involuntary movements have been observed in some patients who received fetal implants. A limitation of stem cell therapy is that it will only affect those aspects of PD that are dependent on dopamine.
Gene therapy
Gene delivery of the growth factor analogue adeno-associated type-2 vector (AAV2)-neurturin has been investigated in patients with advanced PD. When surgically placed inside a neuron, neurturin enhances neuron vitality, enabling it to better fight oxidative stress and other attacks. It fared no better than sham surgery on changes in UPDRS motor score at 12 months in a randomized trial.22 A few patients enrolled in this trial have been followed for longer than 12 months, at which time the mean change in motor scores appears to favor the group assigned to gene delivery of AAV2-neurturin. A phase 1/2 trial is investigating the safety and efficacy of bilateral intraputaminal and intranigral administration of neurturin.
SUMMARY
Levodopa is a legitimate choice for the treatment of early PD. Two MAO-B inhibitors, rasagiline and selegiline, have a symptomatic effect.
Long-acting oral and transdermal dopamine agonists are effective symptomatic therapies, but they also have an interesting array of side effects, making levodopa a reasonable alternative treatment sooner or later despite its dyskinetic effect. Potential neuroprotective effects remain to be identified.
Amantadine is sometimes overlooked as an option for treating early PD, but it has some special side effects including leg edema, livedo reticularis, and corneal edema. Amantadine does not cause orthostatic hypotension and is free of the side effects of excessive diurnal somnolence and impulse control disorders that are prevalent with dopamine agonists.
In the future, partial dopamine agonists and adenosine antagonists may provide us with additional symptomatic therapies. CoQ10, creatine, calcium channel blockers, and inosine, as well as NSAIDs, are being actively studied as potential disease-modifying agents. Further studies are likely to come from the use of NSAIDs.
Early DBS is a new avenue of investigation as a potential disease modifier. Stem cells are still being studied and limitations of sufficient production and potential tumor growth, among others, have delayed the institution of clinical trials. Gene therapy is an interesting additional treatment modality in active research.