Managing the patient with newly diagnosed Parkinson disease
ABSTRACTThe treatment of early Parkinson disease (PD) is generally symptomatic, although therapy that also offers neuroprotection in early-stage PD would be welcomed. Levodopa remains the most effective agent for relief of PD symptoms, but chronic levodopa therapy is associated with motor fluctuations and dyskinesias, and clinicians may therefore opt to postpone its use. Alternatives to levodopa in early PD include monoamine oxidase (MAO)-B inhibitors, amantadine, and dopamine agonists. MAO-B inhibitors have only mild symptomatic effects. Amantadine is associated with improvement in functional disability and, in a subset of PD patients, a robust symptomatic improvement. Dopamine agonists improve symptoms and may have a neuroprotective effect. Partial dopamine agonists, adenosine A2A-receptor antagonists, and safinamide are symptomatic therapies that are under investigation. Neuro protective strategies under study include enhancement of mitochondrial function, antiinflammatory mechanisms, calcium channel blockade, and uric acid elevation. Deep brain stimulation may slow cognitive and motor decline when used in early PD. Stem cell therapy and gene therapy are still under investigation.
Parkinson disease (PD) is a slowly progressive neurodegenerative disorder. Early PD, or stage 1 or 2 on the Unified Parkinson’s Disease Rating Scale (UPDRS), is characterized by mild symptoms, minimal to mild disability, and lack of postural instability or cognitive decline. The goal of therapy in PD is to help patients retain functional independence for as long as possible. Therapeutic choices in early PD are guided by the effect of symptoms on function and quality of life, consideration of complications associated with long-term levodopa, the likelihood of response fluctuations to levodopa, and the potential for a neuroprotective effect.
SYMPTOMATIC THERAPIES IN EARLY PD
Dopaminergic replacement therapy with levodopa is a legitimate choice for the treatment of early PD. Use of carbidopa-levodopa has been shown to slow the progression of PD in a dose-dependent manner as evidenced by a decrease in total score on the UPDRS in patients with early PD who were randomly assigned to receive carbidopa-levodopa compared with those who received a placebo.1
Alternatives to levodopa
There are several reasons to choose an alternative to levodopa for the treatment of early PD. The first is to postpone the development of levodopa-induced dyskinesias, which are linked to duration of levodopa treatment and total exposure to levodopa. The second is postponement of the “wearing-off” effect; that is, the reemergence of symptoms that occurs in some patients before their next scheduled dose of levodopa. Such reasoning applies to early PD patients with minimal or no disability and—in particular—to young-onset PD patients who tend to develop vigorous dyskinesias and dramatic wearing-off phenomena. Pharmacologic alternatives to levodopa in early PD include monoamine oxidase (MAO)-B inhibitors, amantadine, and dopamine agonists.
In a placebo-controlled study of selegiline in de novo early-phase PD, Pålhagen et al showed that selegiline monotherapy delayed the need for levodopa. When used in combination with levodopa, selegiline was able to slow the progression of PD as measured by the change in UPDRS total score.3
Amantadine. In an early study of 54 patients with PD, functional disability scores improved significantly with administration of amantadine 200 to 300 mg/d compared with placebo.4 A small subset of patients, perhaps 20% or less, who are treated with amantadine experience robust symptom improvement. Side effects of amantadine include hallucinations, edema, livedo reticularis, and anticholinergic effects. A more recently discovered potential side effect is corneal edema.
Dopamine agonists. Pramipexole (immediate-release [IR] and extended-release [ER]), and ropinirole IR and ER are dopamine agonists that have demonstrated disease-modifying effects and efficacy in improving PD symptoms.
Pramipexole ER administered once daily in early PD was shown to be superior to placebo on the mean UPDRS total score.5 Ropinirole ER produced mean plasma concentrations over 24 hours similar to those achieved with ropinirole IR, and showed noninferiority to ropinirole IR on efficacy measures in patients with de novo PD.5
The effective dosage range of pramipexole ER in early PD is 0.375 to 4.5 mg/d. Side effects include hallucinations, edema, excessive diurnal somnolence, and impulse control disorders (ie, pathologic gambling, hypersexuality, excessive craving for sweets). Compared with pramipexole IR, compliance is enhanced with the ER formulation because of ease of administration, but this formulation also is more expensive.
In early PD, the effective dosage range of ropinirole ER is 8 to 12 mg/d.6 The side effects are the same as with pramipexole ER with the same compliance advantage and cost disadvantage compared with the IR formulation.
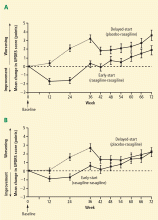
Research indicates that dopamine agonists may have a neuroprotective effect. In two large clinical trials in which patients with PD were followed with an imaging marker of dopamine neuronal degeneration (using single-photon emission computed tomography or positron emission tomography), recipients of pramipexole7 or ropinirole8 showed slower neuronal deterioration compared with levodopa recipients. A counterargument to the neuroprotective theory is that these differences between the dopamine agonists and levodopa reflect neurotoxicity of levodopa rather than neuroprotection by dopamine agonists. The absence of a placebo comparison in both trials adds to the difficulty in drawing a conclusion, as some critics ascribed the differences between groups to downregulation of tracer binding with levodopa.
Nonergoline dopamine agonist. Transdermal rotigotine is a nonergot D1/D2/D3 agonist. Higher doses produce higher plasma levels of rotigotine, which remain steady over the 24-hour dosing interval.9 Transdermal rotigotine has demonstrated effectiveness in early PD in several clinical trials.10,11 The patch, applied once daily, provides a constant release of medication. Removing the patch immediately interrupts drug administration.
Rotigotine patches must be refrigerated to prevent crystallization, a requirement that has delayed the product’s arrival on the market. The patch is reputed to be difficult to peel from its backing and apply. Skin reactions are a side effect, and nonergot side effects are possible. Despite these drawbacks, transdermal rotigotine represents a convenient option for perioperative management of PD and in patients with dysphagia.
Exercise. Exercise has symptomatic and possibly neuroprotective benefits in PD, supporting its use as an additional medical measure. Evidence supports the value of treadmill walking and high-impact exercise in improving stride length, quality of life, and motor response to levodopa.