Skin and soft-tissue infections: Classifying and treating a spectrum
ABSTRACTSkin and soft-tissue infections (SSTIs) are a common presenting problem in both inpatients and outpatients. SSTIs have been broadly classified as complicated or uncomplicated, but specific disease processes and patient characteristics are important in guiding clinical management. Early recognition of the extent of infection, close follow-up, and familiarity with local antibiotic susceptibility data are critical to successful treatment.
KEY POINTS
- Categories and definitions of specific subtypes of infections are evolving and have implications for treatment.
- Methicillin-resistant Staphylococcus aureus (MRSA) and streptococci continue to be the predominant organisms in SSTIs.
- A careful history and examination along with clinical attention are needed to elucidate atypical and severe infections.
- Laboratory data can help characterize the severity of disease and determine the probability of necrotizing fasciitis.
- Although cultures are unfortunately not reliably positive, their yield is higher in severe disease and they should be obtained, given the importance of antimicrobial susceptibility.
- The Infectious Diseases Society of America has recently released guidelines on MRSA, and additional guidelines addressing the spectrum of SSTIs are expected within a year.
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
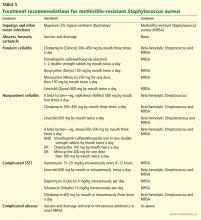
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32