History and current concepts in the pathogenesis of PML
ABSTRACTThe JC virus (JCV), first described in 1971, is responsible for initiation of progressive multifocal leukoencephalopathy (PML), a disease characterized by demyelinating plaques and a classic triad of symptoms consisting of cognitive impairment, visual deficits, and motor dysfunction. To establish a diagnosis of PML, evidence of the presence of JCV DNA in pathologic tissue is necessary. The host range for productive infection of JCV is controlled by factors in the cell nucleus that bind to the viral promoter, initiating transcription of mRNA for the coordinated synthesis of viral proteins. Oligodendrocytes, astrocytes, and CD34+ and CD19+ cells of the immune system have the necessary binding proteins in sufficient concentration to allow lytic infection to occur. A strong link between JCV infection in cells of the immune system and cells of the nervous system points to the importance of the tissue origin of JCV latency, the bone marrow that harbors CD34+ cells. The emergence of PML in patients treated with natalizumab and other immune-altering agents supports this observation and provides new insights into the pathogenic mechanisms of JCV infection.
Susceptibility is related to nucleotide sequences
Susceptibility to PML is associated with promoter/enhancer nucleotide sequences. The tandem repeat nucleotide structure has been found in the peripheral blood leukocytes and the CSF of patients with PML. Although the arrangement of nucleotide sequences in the viral regulatory region is highly variable among patients with PML, there are no alterations in the sequence within the origin of DNA replication. These highly conserved sequences contain regions for DNA-binding proteins that drive transcription, initiating the life cycle of the virus.
The nuclear transcription factor NF-1 is a cell-specific regulator of JCV promoter/enhancer activity. In humans, the NF-1 family of DNA-binding proteins is encoded by four discrete genes, one of which is NF-1 class X (NF-1X), a critical transcription factor that affects JCV cells. The human brain makes NF-1X in concentrations greater than the concentrations of other NF-1 transcription family members of DNA-binding proteins. NF-1X is located adjacent to and interacts with another family of transcription factors, activator protein-1, which has also been associated with JC viral activity.
Spi-B expression a factor in natalizumab-treated patients
Another transcription factor, Spi-B, binds to sequences present in the JCV promoter/enhancer. Spi-B is a regulator of JCV gene expression in susceptible cells and appears to play an important role in JCV activity. The expression of Spi-B is upregulated in patients with MS who are treated with the monoclonal antibody natalizumab, a population of patients in whom PML has been recently described.11–15
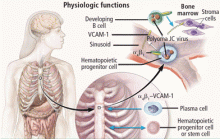
Natalizumab binds to the alpha-4 integrin molecule, preventing hematopoietic stem cells and developing B cells from attaching to a vascular-cell adhesion molecule and forcing them to migrate from the bone marrow (Figure 2).16 An ideal environment is created for JCV when the natalizumab-induced increase in CD34+ cells in the circulation is combined with upregulation of gene cells involved in B-cell maturation. JCV can reside in the bone marrow in a latent state and can use B cells and their DNA-binding proteins to initiate viral multiplication, eventually gaining entry into the brain to cause PML.
In addition to natalizumab, PML has been described in patients treated with efalizumab, another biologic agent that binds alpha-4 integrin molecules on the surface of T and B cells, preventing their entry into the brain, gut, and skin, and forcing migration of bone marrow CD34+ into peripheral circulation for long periods.9,17,18 Rituximab, another monoclonal antibody, binds the CD20 surface molecule on B cells, causing their depletion from the peripheral circulation through complement-mediated cytolysis.7
Risk factors for development of PML
Measurable risk factors for PML include:
- Rising antibody titers
- Evidence of viremia, especially persistent viremia associated with repeat sequences in the regulatory region of the viral genome
- Ineffective T-cell (CD4 and CD8) responses
- Molecular host factors (ie, Spi-B expression in B cells) that support JCV infection in potentially susceptible cells.
The presence of more than one of these risk factors is necessary for development of PML.
VIRAL LATENCY IN B LYMPHOCYTES IN BONE MARROW
A strong link between JCV infection in cells of the immune system and those of the nervous system points to the importance of the tissue origin of JCV latency. Bone marrow harbors CD34+ cells that migrate into the peripheral circulation and undergo differentiation to pre-B and mature B cells, augmenting JCV growth. The emergence of PML in patients treated with natalizumab, rituximab, efalizumab, and other immune-altering drugs underscores this observation.
As noted, the incidence of PML in natalizumab-treated patients with MS and Crohn disease rises as the number of doses increases. Analysis of blood samples collected from patients treated with natalizumab at baseline and again during treatment at months 1 to 12 and beyond 24 months demonstrates that the frequency of CD34+ cells in the peripheral circulation increases with the duration of therapy, adding credence to the theory that CD34+ cells act as a reservoir for latent virus. A higher frequency of CD34+ cells is associated with viremia.
The role of Spi-B in JC virus latency
Understanding the role of Spi-B during JCV latency and reactivation is increasingly important as the number of patients treated with immunomodulatory agents that can develop PML continues to rise. Spi-B is highly represented in the B cell and CD34+ cell fractions. Spi-B expression in B cells correlates with reactivation of JCV in immune cells in natalizumab-treated patients. In a sample of four patients with MS treated with natalizumab who developed PML, T-cell responses have been ineffective (absent or aberrant). Two patients had no detectable T-cell response to JCV; the other two demonstrated response, but their CD4 T-cell responses were dominated by interleukin-10–producing cells.
Longitudinal examination of CSF samples from 13 MS patients who were treated with natalizumab and subsequently developed PML revealed persistence of viral load even though all patients experienced immune reconstitution inflammatory syndrome and most had high levels of anti-JCV antibodies.19