Electrical vagus nerve stimulation for the treatment of chronic heart failure
ABSTRACTAutonomic dysregulation is a feature of chronic heart failure (HF) and is characterized by a sustained increase of sympathetic drive and by withdrawal of parasympathetic activity. Both sympathetic overdrive and increased heart rate are predictors of poor long-term outcome in patients with HF. Pharmacologic agents that partially inhibit sympathetic activity, such as beta-adrenergic receptor blockers, effectively reduce mortality and morbidity in patients with chronic HF. In contrast, modulation of parasympathetic activation as a potential therapy for HF has received only limited attention because of its inherent complex cardiovascular effects. This review examines results of experimental animal studies that provide support for the possible use of electrical vagus nerve stimulation (VNS) as a long-term therapy for the treatment of chronic HF. The review also addresses the effects of VNS on potential modifiers of the HF state, including proinflammatory cytokines, nitric oxide elaboration, and myocardial expression of gap junction proteins. Finally, the safety, feasibility, and efficacy trends of VNS in patients with advanced HF are reviewed.
VNS in combination with beta-blockade
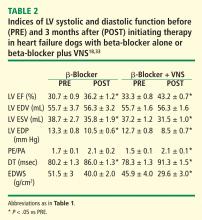
The effects of VNS in combination with beta-blockade were examined in dogs with HF. Dogs with LV ejection fraction of approximately 35% were randomized to 3 months of therapy with a beta-blocker alone (metoprolol succinate, 100 mg once daily, n = 6) or to metoprolol (100 mg once daily) combined with active VNS with CardioFit (n = 6). As with the monotherapy study, the CardioFit VNS system was operated in the feedback on-demand heart rate responsive mode. Dogs were started on oral metoprolol therapy 2 weeks prior to randomization to VNS therapy. After randomization, all dogs continued to receive metoprolol succinate once daily for the duration of the study.18
The improvements in LV systolic and diastolic function with combination therapy were associated with important changes in heart rate. Twenty-four–hour ambulatory ECG Holter monitoring studies showed no differences in minimum and average heart rate between dogs treated with metoprolol and those treated with combination therapy with VNS. Maximum heart rate, however, was significantly lower in dogs treated with the combination therapy (114 ± 12 vs 149 ± 8 beats/min, P < .05). These observations suggest that preventing heart rate escape at the high end may further improve LV systolic function compared with beta-blockade alone. This added benefit of the combination of VNS and beta-blockade was likely the result of reducing the adverse impact of increased cardiac workload and increased myocardial oxygen consumption elicited by the heart rate increase.
VNS and left ventricular remodeling
VNS and proinflammatory cytokines, nitric oxide, and gap junction proteins
Nitric oxide (NO) is formed by a family of NO synthases (NOS). The three isoforms of NOS identified to date are endothelin NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). The three isoforms have differing characteristics and roles:
eNOS. NO produced by eNOS plays an important role in the regulation of cell growth and apoptosis22 and can enhance myocardial relaxation and regulate contractility.22,23
iNOS. Overexpression of iNOS in cardiomyocytes in mice results in peroxynitrite generation associated with fibrosis, LV hypertrophy, chamber dilation, cardiomyopathic phenotype, heart block, and sudden cardiac death.24
nNOS. nNOS has been shown to be upregulated in the human failing heart and in rats following myocardial infarction.25 In rats with HF, inhibition of nNOS leads to increased sensitivity of the myocardium to beta-adrenergic stimulation,26 suggesting a role for nNOS in the autocrine regulation of myocardial contractility.26
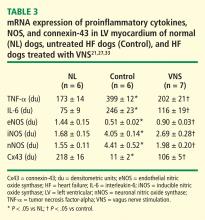
In dogs with coronary microembolization-induced HF, mRNA and protein expression of eNOS in LV myocardium is significantly downregulated compared with normal dogs; therapy with VNS significantly improves the expression of eNOS (Table 3).27 Both iNOS and nNOS are significantly upregulated, and their expression tends to be normalized by long-term VNS therapy.27
Gap junction proteins or connexins are reduced or redistributed from intercalated disks to lateral cell borders in a variety of cardiac diseases, including HF.28 This so-called “gap junction remodeling” is considered highly arrhythmogenic. In mammals, gap junctions exclusively contain connexin-43 (Cx43). Reduced expression of Cx43 occurs in the failing human heart and has been shown to result in slowed transmural conduction and dispersion of action potential duration with increased susceptibility to arrhythmia and sudden cardiac death.29,30 In dogs with coronary microembolization-induced HF, mRNA and protein expression of Cx43 in LV myocardium was shown to be markedly downregulated compared with normal dogs, and long-term therapy with VNS was associated with a significant increase in the expression of Cx43 in LV myocardium (Table 3).31