Electrical vagus nerve stimulation for the treatment of chronic heart failure
ABSTRACTAutonomic dysregulation is a feature of chronic heart failure (HF) and is characterized by a sustained increase of sympathetic drive and by withdrawal of parasympathetic activity. Both sympathetic overdrive and increased heart rate are predictors of poor long-term outcome in patients with HF. Pharmacologic agents that partially inhibit sympathetic activity, such as beta-adrenergic receptor blockers, effectively reduce mortality and morbidity in patients with chronic HF. In contrast, modulation of parasympathetic activation as a potential therapy for HF has received only limited attention because of its inherent complex cardiovascular effects. This review examines results of experimental animal studies that provide support for the possible use of electrical vagus nerve stimulation (VNS) as a long-term therapy for the treatment of chronic HF. The review also addresses the effects of VNS on potential modifiers of the HF state, including proinflammatory cytokines, nitric oxide elaboration, and myocardial expression of gap junction proteins. Finally, the safety, feasibility, and efficacy trends of VNS in patients with advanced HF are reviewed.
Autonomic imbalance characterized by sustained sympathetic overdrive and by parasympathetic withdrawal is a key maladaptation of the heart failure (HF) state. This autonomic dysregulation has long been recognized as a mediator of increased mortality and morbidity in myocardial infarction and HF.1,2 Sympathovagal imbalance in HF can lead to increased heart rate, excess release of proinflammatory cytokines, dysregulation of nitric oxide (NO) pathways, and arrythmogenesis. Diminished vagal activity reflected in increased heart rate is a predictor of high mortality in HF.3,4 Sustained increase of sympathetic activity contributes to progressive left ventricular (LV) dysfunction in HF and promotes progressive LV remodeling.5,6 Pharmacologic agents that reduce heart rate, such as beta-blockers and, more recently, specific and selective inhibitors of the cardiac pacemaker current If, have been shown to improve survival and prevent or attenuate progressive LV remodeling in animals with HF.4,5,7,8
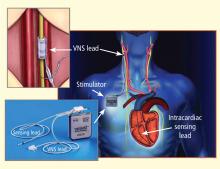
During the past two to three decades, the emphasis on modulation of neurohumoral activition for treatment of chronic HF gave rise to angiotensin-converting enzyme inhibitors, beta-adrenergic receptor blockers, and aldosterone antagonists. In recent years, renewed interest has emerged in modulating parasympathetic or vagal activity as a therapeutic target for treating chronic HF. An alteration in cardiac vagal efferent activity through peripheral cardiac nerve stimulation can produce bradycardia and can modify atrial as well as ventricular contractile function.9,10
Electrical vagus nerve stimulation (VNS) was shown to prevent sudden cardiac death in dogs with myocardial infarction and to improve long-term survival in rats with chronic HF.11,12 VNS has also been shown to suppress arrhythmias in conscious rats with chronic HF secondary to myocardial infarction.13
This article focuses primarily on the effects of chronic VNS on LV dysfunction and remodeling in dogs with HF produced by multiple sequential intracoronary microembolizations14 or by high-rate ventricular pacing15 and on the safety, feasibility, and efficacy trends of VNS in patients with advanced HF.16
VNS IN DOGS WITH MICROEMBOLIZATION-INDUCED HEART FAILURE
Monotherapy with VNS
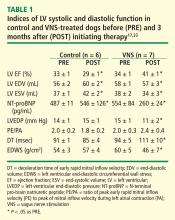
Long-term VNS therapy also elicited improvements in indices of LV diastolic function. VNS significantly decreased LV end-diastolic pressure (Table 1), increased deceleration time of rapid mitral inflow velocity, tended to increase the ratio of peak mitral inflow velocity during early LV filling to peak mitral inflow velocity during left atrial contraction (PE/PA), and significantly reduced LV end-diastolic circumferential wall stress, a determinant of myocardial oxygen consumption (Table 1). These measures suggest that VNS can reduce preload, improve LV relaxation and improve LV function without increasing myocardial oxygen consumption.