Cardiovascular implantable electronic device infection: A stepwise approach to diagnosis and management
ABSTRACTInfection related to cardiovascular implantable electronic devices is a serious complication, necessitating removal of the device and prolonged parenteral antibiotic therapy. Accurate diagnosis and optimal management of these infections are challenging. This review highlights the critical management decisions.
KEY POINTS
- Although inflammatory signs at the generator pocket are the most common presentation of an infection occurring soon after the device is implanted, positive blood cultures may be the sole manifestation of a late-onset endovascular infection.
- Staphylococci are the most common pathogens in both pocket infections and endovascular infections.
- Two sets of blood cultures should be obtained in all patients suspected of having a cardiac device infection.
- Transesophageal echocardiography should be ordered in all patients with suspected cardiac device infection who have positive blood cultures, as it can identify intracardiac complications of infection and assess for evidence of cardiac valve involvement.
A STEPWISE APPROACH TO MANAGING DEVICE INFECTION
Should antibiotics be started empirically?
The first step in managing CIED-related infection is to decide whether empiric antibiotic therapy should be started immediately once infection is suspected or if it is prudent to wait until the culture results are available.
In our opinion, if the infection is limited to the generator pocket, it is reasonable to wait until immediately before surgery to maximize the culture yield from pocket tissue samples. An exception to this rule is when systemic signs or symptoms are present, in which case delaying antibiotic therapy could jeopardize the outcome (FIGURE 2). In such cases, empiric antibiotic therapy can be started once two sets of peripheral blood samples for cultures have been obtained.
Which antibiotics should be given empirically?
Because gram-positive organisms, namely coagulase-negative staphylococci and S aureus, are the causative pathogens in most cases of CIED-related infection, empiric antibiotic therapy should provide adequate coverage for these organisms. Because methicillin resistance is quite prevalent in staphylococci, we routinely use vancomycin (Vancocin) for empiric coverage. In patients who are allergic to vancomycin or cannot tolerate it, daptomycin (Cubicin) is an alternative.
Empiric gram-negative coverage is generally reserved for patients who present with systemic signs and symptoms, in whom delaying adequate coverage could have untoward consequences. We routinely use cefepime (Maxipime) for empiric gram-negative coverage in our institution. Other beta-lactam agents that provide coverage for gram-negative bacilli, especially Pseudomonas, are also appropriate in this setting.
Should the device be removed?
Superficial infection of the wound or incision site (eg, stitch abscess) early after implantation can be managed by conservative antibiotic therapy without removing the device. However, complete removal of the device system, including intracardiac leads, is necessary in all other presentations of device infection, even if the infection appears limited to the generator pocket.5,12 Leaving the device in place or removing parts of the device is associated with persistent or relapsed infection and is not advisable.17,26
Leaving the device in place may be necessary in extenuating circumstances, eg, if surgery would be too risky for the patient or if the patient refuses device removal or has a short life expectancy. In these cases, lifelong suppressive antibiotic therapy should be prescribed after an initial course of parenteral antibiotics.27 Antibiotic choices for long-term suppressive therapy should be guided by antimicrobial susceptibility testing and consultation with an infectious disease specialist.
How should the leads be removed?
Leads are extracted percutaneously in most cases. Percutaneous extraction is generally considered safe even in cases in which infection is complicated by lead vegetations, which raises concern about pulmonary embolization of detached vegetation fragments during extraction.5,20
Thoracotomy is generally reserved for patients who have cardiac complications (such as a cardiac abscess or the need to replace cardiac valves) or in whom attempts to extract the leads percutaneously are unsuccessful.
Details of the removal procedure and choice of extraction technique are beyond the scope of this paper and are best left to the discretion of the treating cardiologist or cardiac surgeon. Because of the potential for complications during percutaneous device removal, such as laceration of the superior vena cava or cardiac tamponade, the patient should be referred to a high-volume center where cardiothoracic intervention can be provided on an emergency basis if needed.
How long should antibiotic therapy go on?
An algorithm for deciding the duration of antibiotic therapy is shown in Figure 3. These guidelines, first published in 2007,17 were adopted by the American Heart Association in its updated statement on the management of CIED-related infections.5 However, it should be noted that these guidelines are not based on randomized clinical trials; rather, they represent expert opinion based on published series of patients with CIED-related infections.
In general, cases of device erosion or pocket infection can be treated with 1 to 2 weeks of appropriate antibiotic therapy based on antimicrobial susceptibility testing. However, cases of bloodstream infection require 2 to 4 weeks of antibiotic therapy—or sometimes even longer if associated complications are present, such as septic thrombosis, endocarditis, or osteomyelitis.
We favor parenteral antibiotics for the entire course of treatment. However, patients can be discharged from the hospital once the bloodstream infection has cleared, and the antibiotic course can be completed on an outpatient basis.
Outpatient antimicrobial monitoring
We recommend adherence to the Infectious Diseases Society of America’s guidelines for monitoring outpatient parenteral antimicrobial therapy.28
At discharge from the hospital, patients should be instructed to promptly call their primary care physician if they have a fever or notice inflammatory changes at the pocket site. If the patient reports such symptoms, repeat blood cultures should be ordered, and the patient should be monitored closely for signs of a relapse of infection.
A routine follow-up visit should be arranged at 2 weeks and at the end of parenteral antibiotic therapy (for patients receiving therapy for 4 weeks or longer) to make sure the infection has resolved.
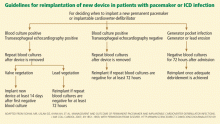
When should a new device be implanted?
Before deciding when a new device should be implanted, one should carefully assess whether the patient still needs one. Studies indicate that up to 30% of patients may no longer require a cardiac device.17,18
However, we believe that removal of drains and closure of the old pocket are not necessary before implanting a new device in a different location (usually the contralateral pectoral area). Exceptions to this general principle are cases of valvular endocarditis, in which a minimum of 2 weeks is recommended between removal of an infected device (plus clearance of bloodstream infection) and implantation of a new device.