Hepatitis C virus: Prevention, screening, and interpretation of assays
ABSTRACTPatients at risk of hepatitis C virus (HCV) infection should be screened for it so that they can be treated and potentially cured, or can at least avoid transmitting the disease to others. The authors describe why and how to screen for HCV and how to interpret the test results.
KEY POINTS
- Patients who should be screened include intravenous drug abusers, people infected with human immunodeficiency virus, patients with unexplained elevated alanine aminotransferase levels, infants born to infected mothers, and people with infected sexual partners.
- Patients at risk of HCV infection should be tested for anti-HCV antibody using an enzyme immunoassay (EIA).
- Positive results on anti-HCV EIA testing should be confirmed with an assay for HCV RNA.
- HCV genotyping can help predict the response to therapy. Genotypes 2 or 3 are more likely to respond to therapy than genotype 1.
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
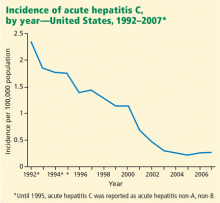
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.