Vaccine update 2010: Keeping up with the changes
ABSTRACTA number of new vaccines have been approved in recent years, and existing vaccines have been extended to new groups. We summarize the additions and changes over the past 3 years.
KEY POINTS
- New recommendations for infants and children:
- Rotavirus vaccination for infants
- Seasonal influenza vaccine yearly at ages 5–18
- Hepatitis A vaccine at age 12–23 months
- Varicella vaccine at 12–15 months and again at 4–6 years, with catch-up for others.
- New recommendations for adolescents:
- Meningococcus quadrivalent conjugate vaccine for all at age 11 or 12 and catch-up through age 18
- A shot of tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis vaccine (Tdap) at age 11 or 12 and catch-up through age 18
- Human papillomavirus vaccine (three doses) for girls at age 11 or 12 and catch-up through age 26.
- New recommendations for adults:
- One dose of Tdap instead of the next tetanus-diphtheria booster
- Herpes zoster vaccine at age 60 or older
- Pneumococcal vaccination extended to smokers and people with asthma, with a second dose 5 years after the first for people who have immune suppression, sickle cell disease, or asplenia.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
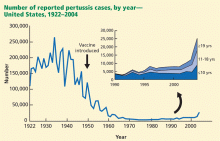
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.