Managing gout: How is it different in patients with chronic kidney disease?
ABSTRACTMany patients with gout have comorbidities, including hypertension and chronic kidney disease (CKD). The goals when treating gout are no different in these patients, but the choice and dosage of drugs may need to be modified.
KEY POINTS
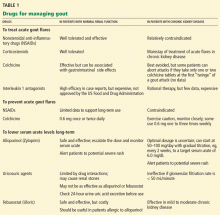
- Owing to concerns about using colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs) in patients with CKD, glucocorticoids (local injections or systemic therapy) are often used to treat acute attacks. Corticotropin (Acthar), anti-tumor necrosis factor agents, and interleukin 1 antagonists are effective but expensive.
- Colchicine can be used in low doses as prophylaxis, with caution and appropriate monitoring. NSAIDs should be avoided, and glucocorticoids may not be effective for this purpose.
- Whether the dosage of allopurinol should be lower in patients with CKD remains controversial. We start with a low dose and slowly increase it, with a goal serum urate level of less than 6.0 mg/dL.
- Febuxostat (Uloric), like allopurinol, is a xanthine oxidase inhibitor, but the elimination of the active drug is not by the kidney. Nevertheless, we try allopurinol in escalating doses first, due to major cost differences.
Corticosteroids are often used to treat acute attacks
Due to the concerns about NSAIDs or colchicine to treat acute gout attacks in patients with CKD, corticosteroids are often used in this setting.
Intra-articular steroid injections are useful in treating acute gout limited to a single joint or bursa.12 However, one should first make sure that the joint is not infected: septic arthritis should ideally be excluded by arthrocentesis, particularly in immunosuppressed patients13 or those with end-stage renal disease, who are predisposed to bacteremia.
Oral, intramuscular, or intravenous steroids can provide complete relief from acute gout, although high doses (eg, prednisone 30–60 mg/day or the equivalent) are often needed. Common errors resulting in inefficacy include using too low a dose or not treating for a sufficient time before tapering or stopping. Groff and colleagues14 described 13 patients who received oral or intravenous steroids for acute gout. Nine patients received an initial single dose of prednisone ranging from 20 to 50 mg, with tapering over a mean of 10 days. Twelve of the 13 patients had improvement within 48 hours, and the signs and symptoms of acute gout resolved completely within 7 to 10 days.
We often give prednisone 40 mg daily until a day after the acute attack resolves and then taper over another 7 to 10 days. There are no data to guide steroid dosing in an evidence-based way, but we believe too short a course of therapy may result in return of symptoms.
Corticotropin and other agents: Effective but costly
Corticotropin is available for subcutaneous or intramuscular injection. A single intramuscular injection of corticotropin gel (H.P. Acthar, 25–80 IU) may terminate an acute gout attack.15 However, many patients need another injection after 24 to 72 hours, which would require another visit to the physician. This treatment has been touted by some as being more effective than corticosteroid therapy, possibly because of a unique peripheral mechanism of action in addition to stimulating cortisol release.16
We rarely use corticotropin, in view of its cost as well as concerns about excessive sodium and water retention due to the release of multiple hormones from the adrenal gland. This may be especially deleterious in patients with CKD or congestive heart failure.17
Parenteral anti-tumor necrosis factor agents or interleukin 1 antagonists can be dramatically effective but are also expensive.18,19 For example, anakinra (Kineret) 100 mg costs about $73, and multiple daily doses may be necessary.
Under unique conditions in which they can be safely used (eg, patients with CKD, diabetes mellitus, liver disease), they may be cost-effective if they can shorten the stay of a hospitalized patient with acute gout.
PROPHYLACTIC ANTI-INFLAMMATORY THERAPY FOR PATIENTS WITH GOUT
Between attacks, the goal is to prevent new attacks through prophylactic management, which may include anti-inflammatory and hypouricemic therapy along with dietary instruction (such as avoiding excessive beer, liquor, and fructose ingestion).
Colchicine can be used as prophylaxis, with caution and monitoring
Although colchicine is not 100% effective, it markedly reduces the flare rate when started in low doses at the time hypouricemic therapy is initiated.20,21 (Hypouricemic therapy is discussed below.) We generally try to continue this prophylactic therapy, if the patient tolerates it, for at least 6 months—longer if tophi are still present or if attacks continue to occur.
If renal function is intact, colchicine can be prescribed at a dosage of 0.6 mg orally once or twice daily.21 In CKD, since the clearance of colchicine is reduced,4 the dosage should be reduced. Patients on colchicine for prophylaxis must be carefully monitored if the glomerular filtration rate is less than 50 mL/minute, or colchicine should be avoided altogether.6 Laboratory testing for colchicine levels is not routinely available and may be of limited value in predicting adverse effects; thus, recommendations about dose adjustments in CKD are empiric.
Wallace et al22 recommended a dose of 0.6 mg once daily if the creatinine clearance is 35 to 49 mL/minute and 0.6 mg every 2 to 3 days if it is 10 to 34 mL/minute, but there are no published long-term safety or efficacy data validating these reasonable (based on available information) dosing regimens.
Even with dose adjustment, caution is needed. Low-dose daily colchicine may be associated with reversible neuromyopathy and bone marrow suppression.7,23 Patients with neuromyopathy may complain of myalgias, proximal muscle weakness, and numbness and may have areflexia and decreased sensation. Laboratory findings include elevated creatine kinase and aminotransferase levels. We regularly check for leukopenia or elevated creatine kinase and aspartate aminotransferase levels in patients with CKD who are receiving colchicine in any dose.
Prolonged colchicine therapy should probably be avoided in patients on hemodialysis, as this drug is not removed by dialysis or by exchange transfusion, and the risk of toxicity under these circumstances may be high.22 When there is no viable alternative and the drug is given, patients should be closely monitored for signs of toxicity.
Concurrent (even short-term) treatment with most macrolide antibiotics, particularly clarithromycin (Biaxin), most statin drugs, ketoconazole (Nizoral), cyclosporine, and likely other drugs predisposes to colchicine toxicity by altering its distribution and elimination, and can in rare cases cause morbidity or death.24–26
NSAIDs are not optimal as prophylaxis in patients with chronic kidney disease
Little information has been published about using NSAIDs chronically to prevent flares, but they are not the optimal drugs to use in patients with CKD, as discussed above. In patients with end-stage renal disease, there are also concerns about NSAID-induced gastric and intestinal bleeding.
Low-dose steroids may not be effective as prophylaxis
Lower doses of steroids may not be effective as prophylaxis against gout flares, consistent with the common observation that gout flares still occur in organ transplant recipients who are taking maintenance doses of prednisone.13