When ‘blue babies’ grow up: What you need to know about tetralogy of Fallot
ABSTRACTMost babies born with tetralogy of Fallot undergo corrective surgery and survive to adulthood. However, as they get older they are prone to a number of long-term problems, and they often do not receive expert-level follow-up care. This review of the adult complications of tetralogy of Fallot should help primary care practitioners identify these patients, make appropriate and timely referrals, and educate patients and their families.
KEY POINTS
- The major long-term complication of tetralogy of Fallot repair is pulmonary valve insufficiency, which leads to right heart failure. Other problems include atrial and ventricular arrhythmias and sudden cardiac death.
- Surgical pulmonary valve replacement is the standard of care, but the optimal time to do this is unclear.
- Novel and experimental therapies include percutaneous pulmonary valve replacement and medical therapy with pulmonary arterial vasodilators.
Imaging studies
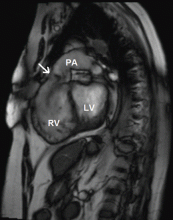
Chest radiography typically shows a prominent right ventricular shadow and cardiomegaly.17
Many centers specializing in congenital heart disease therefore recommend baseline cardiac MRI, even for patients without symptoms.33
PULMONARY VALVE REPLACEMENT IS THE ONLY PROVEN TREATMENT
No study has yet shown that drug therapy alone slows the progression of complications.1 Pulmonary valve replacement is the only treatment proven to reduce right ventricular size and improve right ventricular function in the long term.
The risks of surgery, including the need for repeat operations, must be balanced against the risk of irreversible right ventricular dysfunction and its associated complications. The operative death rate is low, as is the long-term risk of death afterward. Therrien et al12 reported that, in a series of 70 patients who underwent pulmonary valve replacement, the probability of survival was 92% at 5 years and 86% at 10 years.
Surgery appears to reverse or at least arrest the progression of many of the complications associated with pulmonary insufficiency, including tricuspid regurgitation and diastolic dysfunction.17 Its utility in ameliorating ventricular tachycardia, however, remains controversial. One series showed a lower prevalence of tachycardia after pulmonary valve replacement (9% after surgery vs 22% before), but later studies have had more equivocal results.17
When should surgery be done?
There is little controversy about the eventual need for pulmonary valve replacement in most patients. What is controversial is the timing.12,44–47
This issue has been hotly debated. Some believe that pulmonary valve replacement should be done only if evidence of right ventricular dysfunction has developed.17 Others suggest that it be considered earlier and that the onset of symptoms may be a late and suboptimal indication for it.6,8,48,49 Many experts now recommend surgery early, before symptoms of heart failure develop.17 Though surgery has traditionally been recommended if the QRS duration is longer than 180 ms, some believe it should be done before this occurs.11
Arguments for early surgery. In one study, in no patient who had a right ventricular end-diastolic volume greater than 170 mL/m2 (normal ≤ 108) or a right ventricular end-systolic volume greater than 85 mL/m2 (normal ≤ 47) did these numbers return to normal after pulmonary valve replacement.45,50 This suggests a point of irreversible dilatation and a volume threshold beyond which right ventricular function is unlikely to completely improve. Normalization of right ventricular volumes was shown to occur when pulmonary valve replacement was performed before the right ventricular end-diastolic volume reached 160 mL/m2 or the right ventricular end-systolic volume reached 82 mL/m2.47,51
Delaying surgery until symptoms occur may be unfavorable because the long-term outcomes of increased right ventricular volumes and decreased right ventricular ejection fractions after surgery are not known.
Arguments for watchful waiting. There does not seem to be a threshold above which right ventricular volumes do not decrease after surgery—although they may not decrease to the normal range. Pulmonary valve replacement substantially reduced right ventricular dilatation even in patients with very high right ventricular volumes and right ventricular dysfunction, and resulted in an overall improvement in function (measured by New York Heart Association class).47
Late pulmonary valve replacement rapidly improves right ventricular volumes and improves the effective ejection fraction, although its impact on absolute right ventricular function is not as pronounced. The QRS duration shortened after surgery in those in whom it was 180 ms or longer before surgery, although this appeared to be a transient change.52 The prevalence of ventricular tachycardia declined from 22% to 9% and that of atrial fibrillation or flutter declined from 17% to 12%.17,48
A recent study with long-term follow-up has raised questions about the necessity of aggressive early intervention in tetralogy of Fallot. Sixty-seven patients were followed for as long as 27 years after surgery. Forty-five had severe pulmonary insufficiency and severe right ventricular dilatation, and of those, 28 remained free of symptoms and did not undergo pulmonary valve replacement. The authors found that refraining from pulmonary valve replacement in asymptomatic patients with severe pulmonary insufficiency led to no measurable deterioration in 25 of 28 patients.53
The available data do not support pulmonary valve replacement in young patients with mild or moderate right ventricular dilatation, normal right ventricular systolic function, and no additional risk factors.27
Mechanical vs bioprosthetic replacement valves
Once the decision is made to proceed to surgery, the next step is choosing the type of prosthetic valve.
Mechanical valves pose a risk of thrombosis, requiring life-long anticoagulation. To give warfarin (Coumadin) to younger, active people exposes them to the risk of potentially catastrophic bleeding if trauma were to occur. Women who become pregnant are generally at an increased risk of thrombotic complications due to the hypercoagulable state of pregnancy, but the risk of fetal defects is considerable if they receive warfarin.54–56
Bioprosthetic valves generally come in two varieties: preserved and treated human tissue (homografts) and animal tissue (bovine pericardial or porcine, depending on the size required). These can be implanted as isolated valves or as part of a conduit (valve and surrounding tissue).
Bioprosthetic valves eliminate the need for anticoagulation. However, they are not very durable, especially in younger patients, which is worrisome. An estimated 45% of bioprosthetic valves fail by 10 years,57 thus nearly guaranteeing that an otherwise healthy 40-year-old, for example, will need to undergo at least one repeat surgery, and very likely more.