Autonomic function and prognosis
ABSTRACT
Autonomic nervous system function is assessed in the clinic by measuring resting heart rate, heart rate variability, or heart rate recovery following exercise. Each of these measures is a strong predictor of cardiovascular risk and all-cause mortality in primary and secondary prevention settings. These measures have been used to identify correlates of autonomic nervous system dysfunction at both the patient level (eg, obesity, diabetes, heart failure) and the environmental level (eg, smoking, social stress, air pollution). Future research must determine how to exploit the associations between autonomic system dysfunction and poor prognosis to improve patient outcomes.
First-year medical students are well aware that the autonomic nervous system regulates heart rate and blood pressure along with respiratory and digestive functions. The past 10 to 20 years have seen increased appreciation of the medical relevance of the the autonomic nervous system beyond first-year physiology examinations; even mild disturbances of autonomic nervous system function predict materially worse prognosis.1–3 Researchers have focused on the use of readily available measures, such as heart rate,4 heart rate variability,5 and heart rate recovery,6 to link autonomic nervous system dysfunction with mortality and morbidity.1 In addition, epidemiologists have exploited these tools to identify correlates of autonomic nervous system dysfunction at patient and environmental levels.7 Although it is not yet known how best to incorporate autonomic nervous system measures into routine clinical care, there is increasing excitement about the insights that this work has revealed.
MEASURES OF AUTONOMIC NERVOUS SYSTEM FUNCTION
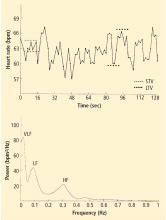
Although many measures of autonomic nervous system function have been described, three relatively straightforward approaches are based on heart rate.1
Resting heart rate is the simplest to obtain, as it does not require any special technology. People with high levels of parasympathetic nervous system tone have lower resting heart rates, as is typically seen in world-class athletes. Conversely, conditions characterized by increased levels of sympathetic tone manifest as sinus tachycardia; classic examples include congestive heart failure, anemia, and hypovolemia.
Heart rate recovery. Heart rate variability measures require continuous Holter monitoring as well as sophisticated software. The numerous types of heart rate variability measures are not intuitive for most clinicians. Exercise heart rate recovery is an arguably more straightforward method of assessing parasympathetic tone.1 During a graded exercise test, heart rate increases as a result of withdrawal of parasympathetic tone and increased sympathetic tone. During the first 30 seconds after exercise, heart rate decreases quickly, mainly because of rapid reactivation of the parasympathetic nervous system.10
AUTONOMIC NERVOUS SYSTEM FUNCTION AND MORTALITY
Resting heart rate
There is a remarkably strong association between heart rate and survival, an association that transcends species.4 Small mammals that have rapid heart rates have short life expectancies. Larger mammals that have slower heart rates have correspondingly higher life expectancies. Among nearly all mammals, life expectancy is close to 1 billion heartbeats.
Investigators have been able to increase survival in animal models by deliberate slowing of heart rate. An experiment performed in mice more than 30 years ago showed that life expectancy increases with low-dose digoxin, a parasympathomimetic agent.11 More recently, a mouse model has been used to show that ivabradine, a sinus node ion channel blocking agent that specifically reduces heart rate without affecting vascular tone, inhibits development of atherosclerosis in genetically susceptible knockout mice.12
There is an extensive epidemiological literature linking heart rate to mortality in large human populations. 4,13 As heart rate increases to 75 to 80 beats per minute, there are marked increases in total mortality and mortality due to coronary heart disease. As is well known, administration of beta-blockers reduces mortality in survivors of myocardial infarction. What is particularly remarkable is that the magnitude of reduction in mortality with beta-blocker therapy is directly proportional to the magnitude of heart rate decrease.14 In a recent analysis of hypertensive patients enrolled in a large-scale randomized trial, a strong association was noted between mortality and increasing heart rate at the time of randomization as well as after treatment with either verapamil or a beta-blocker.15