Managing osteoporosis: Challenges and strategies
ABSTRACTMany patients at high risk of fracture are not being identified and treated, and many of those who start treatment do not take it correctly or long enough to lower their risk. This paper is a review of unmet needs in the management of osteoporosis and strategies to improve clinical outcomes.
KEY POINTS
- Osteoporosis is underdiagnosed. Patients discharged from the hospital after hip fractures are commonly not diagnosed with or treated for osteoporosis although the risk of future fractures is very high.
- The Fracture Risk Assessment Tool (FRAX) estimates the 10-year probability of fracture on the basis of clinical risk factors for fracture and the bone mineral density of the femoral neck. The combination of bone mineral density and clinical risk factors predicts fracture risk better than either alone.
- A drug holiday, for 1 year or perhaps longer, may be considered for patients on alendronate (Fosamax) who are no longer at high risk of fracture. On the other hand, given the evidence of increased risk of clinical vertebral fracture and hip fracture after bisphosphonates are discontinued, a drug holiday is probably not a reasonable choice in patients at high risk of fracture.
- Patient education and regular contact with a health care provider may improve compliance and persistence with therapy.
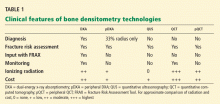
DXA is the gold standard test
Although all of these tests provide results that correlate with fracture risk, DXA is the only one that can be used for diagnostic classification7 and the only one that can be used with the Fracture Risk Assessment Tool, or FRAX (more about this below).22 DXA is also the most clinically useful way to monitor the effects of therapy, with a correlation, albeit an imperfect one, between changes in bone mineral density with therapy and reduction in fracture risk.23
For these reasons, DXA is generally considered the gold standard for measuring bone mineral density.24
Using the wrong technology for the clinical need25 or performing poor-quality testing26,27 may result in inappropriate patient care decisions and wastes limited health care resources.
Medicare coverage for DXA has been cut
Recent cuts in Medicare reimbursement for DXA in the United States have been so severe that payment is now less than the cost of providing the service at many facilities.28 With further reductions in reimbursement expected, it is projected that most outpatient DXA centers—ie, about two-thirds of all DXA facilities in the United States—will no longer be operating by 2010.29
The anticipated consequences: fewer patients will be diagnosed with osteoporosis, fewer patients will be treated, and more fractures will occur, with fracture-related health care expenses far exceeding the savings from fewer DXA tests and fewer prescriptions for drugs to reduce fracture risk. I have characterized this as a “crisis in osteoporosis care,”30 and it is in stark contrast to the mandate of the US Surgeon General to improve osteoporosis care.1
Reports from several large health care systems support the proposition that more rather than fewer patients should undergo bone density testing. Data from the Kaiser Southern California and the Geisinger Health Plan show that when more patients undergo DXA and more are treated for osteoporosis, fewer have fractures, and money is saved.31,32
STRATEGIES FOR IMPROVING DIAGNOSIS
Appoint an advocate
The first step in the early diagnosis of osteoporosis is to select appropriate patients for bone density testing by recognizing high-risk populations.
Given the many demands placed on primary care physicians, who may not be focused on osteoporosis, it may be helpful to appoint one or more office staff as “advocates” for skeletal health. This could be a medical assistant, nurse, or health care educator who is charged with alerting the physician when bone mineral density testing is needed, or who could perhaps be given the authority to order the test within prespecified parameters. Other responsibilities might include patient education on nutrition, lifestyle, fall prevention, and drug administration, and follow-up by phone or in the office to ensure compliance with therapy.
Set up a disease-management program
Changing the health care system may be a more effective way to improve clinical outcomes than changing the actions of individual physicians. Disease-management programs that institutionalize pathways of care for osteoporosis have shown promise,32,33 and postfracture intervention programs may provide an opportunity to better manage patients at very high risk of future fractures.34,35
Lobby your legislators
To assure patient access to diagnostic services for assessment of skeletal health, advocates are focusing on legislation to restore DXA reimbursement to a level that would allow outpatient DXA facilities to avoid financial losses and continue operating.28 This possibility may be aided by grassroots support from concerned physicians and from patients likely to be harmed by limited access to DXA testing because of fewer instruments in operation and greater distances to travel to reach them. The largest US patient advocate organization for osteoporosis care, the National Osteoporosis Foundation (www.nof.org), is spearheading a drive to educate legislators on the value of bone density testing and to pass corrective legislation.
ASSESSING FRACTURE RISK WITH FRAX
The patients who get the greatest reduction in fracture risk with drug therapy are those who have the highest baseline risk of fracture.6 An estimate of fracture risk is therefore important in determining which patients to treat.
While bone mineral density is an excellent predictor of fracture risk, density combined with clinical risk factors for fracture is a better predictor than density or clinical risk factors alone.
FRAX22 is an electronic clinical tool (www.shef.ac.uk/FRAX/) for calculating fracture risk on the basis of the bone mineral density of the femoral neck; the patient’s age, sex, height, and weight; and seven clinical risk factors (previous fracture, having a parent who had a hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, and ingestion of three or more units of alcohol daily). One enters this information plus the brand of DXA machine used (Hologic, GE Lunar, or Norland), and the algorithm estimates the 10-year probability of a major osteoporotic fracture (hip, spine, proximal humerus, or distal forearm), and the 10-year probability of hip fracture
The FRAX model was developed through an analysis of almost 60,000 men and women in 12 population-based cohorts with about 250,000 person-years of observation, and externally validated in an additional 11 cohorts with 230,000 men and women and more than 1.2 million person-years of observation.8 The analysis of these extraordinarily robust databases was a mammoth project undertaken by the World Health Organization, under the direction of Professor John Kanis and with the support of many other organizations and professional societies. Criteria for inclusion of a clinical risk factor in FRAX included international validation, independence from bone mineral density in predicting fractures, ease of collecting the information in clinical practice, and the potential for modification with drug therapy. Falls were not considered as clinical risk factors, since it is not clear that pharmacologic intervention can significantly change the risk of falls.
FRAX remains a work in progress, with continuing updates expected as new information becomes available on country-specific fracture rates and potential additional clinical risk factors. Future versions of FRAX may also include input from skeletal sites other than the femoral neck and bone density measurement with technologies other than DXA.