Anticoagulants and pregnancy: When are they safe?
ABSTRACTPrescribing anticoagulants to pregnant women can be difficult and stressful. Fortunately, low-molecular-weight heparins (LMWHs) and unfractionated heparin are quite safe and efficacious when properly selected, dosed, and monitored. Maternal and fetal concerns must be considered at all times, with a careful assessment of the risks and benefits of anticoagulant therapy in each patient. Further research should help to clarify who should receive thromboprophylaxis, how to prevent adverse pregnancy outcomes in women with various thrombophilias, and how best to treat pregnant women who have a prosthetic heart valve.
KEY POINTS
- Pregnancy is a hypercoagulable state. Thrombotic risk in an individual pregnancy depends on many maternal and situational factors.
- When indicated, careful anticoagulation can proceed with minimal risk to the mother and fetus.
- Heparins, especially LMWHs, are the main anticoagulants used in pregnancy. Dosing depends on the clinical indications and on the agent selected.
- If anticoagulation is absolutely necessary and LMWH is contraindicated, a newer, alternative anticoagulant should be considered.
- Warfarin should not be used in pregnancy in any but the highest-risk situations.
Risks to the fetus and the mother
Unlike the heparins, coumarins freely cross the placenta and thus pose a risk of teratogenicity. A cluster of fetal malformations including “warfarin embryopathy” (nasal bone hypoplasia and chondrodysplasia punctata) can occur when the drug is used between 6 and 12 weeks of gestation. Warfarin embryopathy may be avoided by stopping warfarin prior to 6 weeks from the onset of the last menstrual period (ie, 6-week “menstrual age” or 4-week gestational age34).
Later in pregnancy, warfarin is associated with potential fetal bleeding complications leading to central nervous system abnormalities, increased rates of intrauterine fetal death, and pregnancy loss. In pregnant women with mechanical cardiac valve prostheses who received oral anticoagulants throughout pregnancy, the incidence of congenital anomalies was 6.4% to 10.2%.35 Fetal demise (spontaneous abortion, stillbirth, neonatal death) was also very common (29.7% to 33.6% of pregnancies) in coumarin-treated women.
Severe maternal hemorrhage may also occur in pregnant women on oral anticoagulants, particularly those who remain fully anticoagulated around the time of labor and delivery.
General caveats to warfarin in pregnancy
Because of the many maternal and fetal concerns, oral anticoagulant use in pregnancy is largely restricted to women with older-generation prosthetic heart valves in whom the very high maternal thrombotic risk may outweigh the risk of maternal and fetal side effects.
While there are limited data on warfarin use in pregnant women with antiphospholipid syndrome,36 warfarin use in such patients should be considered only for those at highest risk and with careful informed consent. These issues are discussed further below in the section on mechanical heart valve prostheses.
ANTIPLATELET DRUGS
Aspirin is an antiplatelet agent rather than an anticoagulant. Although considered inadequate for preventing venous thrombosis in high-risk groups when used alone, aspirin can moderately reduce the risk of deep venous thrombosis and pulmonary embolism in nonpregnant patients.37 It also has a well-accepted role in preventing arterial thrombotic events, ie, coronary artery disease and stroke.38
Low-dose aspirin (≤ 100 mg/day) has been extensively evaluated during pregnancy39–41 and has been shown to be safe and effective in reducing the risk of preeclampsia in high-risk women39 and in treating women with antiphospholipid antibodies and recurrent pregnancy loss42 (in conjunction with prophylactic doses of heparin). Although higher doses of aspirin and other nonsteroidal anti-inflammatory drugs can be toxic to the fetus, low doses have been shown to be safe throughout pregnancy.43
Dipyridamole (Persantine) has been studied extensively in pregnancy, and while it appears to be safe, it has not found a well-defined therapeutic role.
Other antiplatelet drugs have been only rarely used, and data on their safety and efficacy during pregnancy are limited to case reports, for example, on ticlopidine44 (Ticlid) and clopidogrel45,46 (Plavix) given during pregnancy in women with cardiac disease. These drugs do not appear to be major teratogens or to cause specific fetal harm. Their use may be reasonable in some high-risk situations, such as recurrent thrombotic stroke despite aspirin therapy. They may be used alone or with other anticoagulants in women with a coronary or other vascular stent if fetal safety is uncertain or if there is an increased risk of maternal bleeding.
NEWER ANTICOAGULANTS
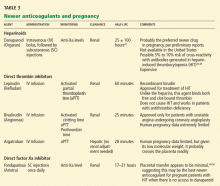
Danaparoid
The heparinoid danaparoid (Orgaran) is an LMWH, a combination of heparan, dermatan, and chondroitin sulfate. Since it is derived from heparin, in theory it can cross-react with antiheparin antibodies, but this is generally not a problem. Danaparoid inhibits factor Xa, and monitoring is via measurement of anti-factor-Xa activity levels. It has been shown to be safe and effective in nonpregnant patients with heparin-induced thrombocytopenia.51
Although no controlled study has been published on danaparoid in pregnancy, at least 51 pregnancies in 49 patients treated with danaparoid have been reported.52 Thirty-two of the patients received danaparoid because of heparin-induced thrombocytopenia and 19 because of heparin-induced skin intolerance. These reports suggest that danaparoid does not cross the placenta53 and that it may be effective and safe during pregnancy.54 For this reason, it is probably the preferred anticoagulant in pregnant patients with heparin-induced thrombocytopenia or other serious reactions to heparin.
Unfortunately, danaparoid has two major disadvantages. First, it has a prolonged half-life and no effective reversing agent, which makes its use problematic close to the time of delivery. Second, and perhaps more relevant to this discussion, it is not readily available in the United States; it was removed from the market by its manufacturer in April 2002 for business reasons rather than because of concerns over toxicity. It is still available in Canada and Europe, and it can be obtained in special circumstances in the United States via the US Food and Drug Administration (FDA); this may be worthwhile in pregnant patients who require a nonurgent alternative to heparin.
Direct thrombin inhibitors
Lepirudin (Refludan), bivalirudin (Angiomax), and argatroban are direct thrombin inhibitors and exert their anticoagulant effect independently of antithrombin. They are given by continuous intravenous infusion, and they have a very short half-life.
Lepirudin and argatroban are typically monitored via the aPTT. Bivalirudin can be monitored with the activated clotting time, partial thromboplastin time, or INR, depending on the circumstances. None of these agents generates or cross-reacts with antibodies generated in heparin-induced thrombocytopenia. None has an antidote, but the short half-life usually obviates the need for one.
Unfortunately, pregnancy data are very sparse for all three of these new agents. Argatroban has a low molecular weight and likely crosses the placenta. Also, because these agents are given intravenously, they are not practical for long-term use in pregnancy.
Fondaparinux
Fondaparinux (Arixtra), a direct factor Xa inhibitor, binds to antithrombin, causing an irreversible conformational change that increases antithrombin’s ability to inactivate factor Xa (as do the heparins). It has no effect on factor IIa (thrombin) and does not predictably affect the aPTT. Its half-life is 17 hours, and no agent is known to reverse its anticoagulant effect, although some experts would recommend a trial of high-dose recombinant factor VIIa (Novo-Seven) in uncontrolled hemorrhage.
While not FDA-approved for treating heparin-induced thrombocytopenia, it has been used for this in some patients.55–58 Animal studies and in vitro human placental perfusion studies suggest that fondaparinux does not cross the placenta in significant amounts.49 Since danaparoid is not available in the United States, fondaparinux would likely be the first choice among the newer anticoagulants when treating heparin-induced thrombocytopenia in pregnancy.