Anticoagulants and pregnancy: When are they safe?
ABSTRACTPrescribing anticoagulants to pregnant women can be difficult and stressful. Fortunately, low-molecular-weight heparins (LMWHs) and unfractionated heparin are quite safe and efficacious when properly selected, dosed, and monitored. Maternal and fetal concerns must be considered at all times, with a careful assessment of the risks and benefits of anticoagulant therapy in each patient. Further research should help to clarify who should receive thromboprophylaxis, how to prevent adverse pregnancy outcomes in women with various thrombophilias, and how best to treat pregnant women who have a prosthetic heart valve.
KEY POINTS
- Pregnancy is a hypercoagulable state. Thrombotic risk in an individual pregnancy depends on many maternal and situational factors.
- When indicated, careful anticoagulation can proceed with minimal risk to the mother and fetus.
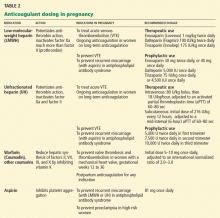
- Heparins, especially LMWHs, are the main anticoagulants used in pregnancy. Dosing depends on the clinical indications and on the agent selected.
- If anticoagulation is absolutely necessary and LMWH is contraindicated, a newer, alternative anticoagulant should be considered.
- Warfarin should not be used in pregnancy in any but the highest-risk situations.
Pregnancy alters metabolism of LMWHs
The physiologic changes of pregnancy alter the metabolism of LMWH, resulting in lower peak levels and a higher rate of clearance,18,19 and so a pregnant woman may need higher doses or more frequent dosing.
Recent evidence suggests that thromboprophylaxis can be done with lower, fixed, once-daily doses of LMWH throughout pregnancy,20 although some clinicians still prefer twice-daily dosing (particularly during the latter half of pregnancy).
Pending more research on weight-based dosing of LMWH in pregnancy, anti-factor- Xa activity levels should be measured after treatment is started and every 1 to 3 months thereafter during pregnancy.21 Doses should be adjusted to keep the peak anti-Xa level (ie, 4 hours after the dose) at 0.5 to 1.2 U/mL.22
Heparin-induced thrombocytopenia
Type-2 heparin-induced thrombocytopenia is an uncommon but serious adverse effect of unfractionated heparin therapy (and, less commonly of LMWH), caused by heparin-dependent immunoglobulin G (IgG) antibodies that activate platelets via their Fc receptors, potentially precipitating life-threatening arterial or venous thrombosis.
In a trial in nonpregnant orthopedic patients,23 clinical heparin-induced thrombocytopenia occurred in 2.7% of patients receiving unfractionated heparin vs 0% of those receiving LMWH; heparin-dependent IgG was present in 7.8% vs 2.2%, respectively.
Fortunately, heparin-induced thrombocytopenia seems to be very rare in pregnancy: two recent prospective series evaluating prolonged LMWH use in pregnancy13,15 revealed no episodes of this disease. Nonetheless, it is reasonable to measure the platelet count once or twice weekly during the first few weeks of LMWH use and less often thereafter, unless symptoms of heparin-induced thrombocytopenia develop. In pregnant women with heparin-induced thrombocytopenia or heparin-related skin reactions, other anticoagulants must be considered24 (see discussion later).
Heparin-induced osteoporosis
Heparin-induced osteoporosis, a potential effect of prolonged heparin therapy, is of concern, given the prolonged duration and high doses of unfractionated heparin often needed to treat venous thromboembolism during pregnancy. Several studies found significant loss of bone mineral density in the proximal femur25 and lumbar spine26 during extended use of unfractionated heparin in pregnancy.
Fortunately, LMWH appears to be much safer with respect to bone loss. Three recent studies27–30 evaluated the use of LMWH for extended periods during pregnancy, and none found any greater loss of bone mineral density than that seen in normal pregnant controls. Giving supplemental calcium (1,000–1,500 mg/day) and vitamin D (400–1,000 IU/day) concomitantly with unfractionated heparin or LMWH in pregnancy is advisable to further reduce the risk.
Interrupt heparin to permit regional anesthesia
Heparin therapy should be temporarily stopped during the immediate peripartum interval to minimize the risk of hemorrhage and to permit regional anesthesia. Because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, many anesthetists will not perform neuraxial regional anesthesia in women who have recently received heparin.
Since unfractionated heparin has a relatively short duration of action, the American Society of Regional Anesthesia states that subcutaneous unfractionated heparin prophylaxis is not a contraindication to neuraxial regional anesthesia.31 However, LMWHs should be stopped for at least 12 to 24 hours before regional anesthesia can be considered safe. This issue is discussed in more detail in the section on peripartum and postpartum management of anticoagulation, below.
In summary, LMWH during pregnancy offers a number of advantages over unfractionated heparin: equivalent efficacy, once- or twice-daily dosing, lower risk of heparin-induced thrombocytopenia and osteoporosis, and less-intensive monitoring. Unfractionated heparin can be offered to women who cannot afford LMWH (which costs four to five times more), and it may be used peripartum to reduce hemorrhagic risk and to permit regional anesthesia.
COUMARINS
Coumarins are the mainstay of anticoagulant therapy in most nonpregnant women beyond the immediate thrombotic period.
Warfarin (Coumadin) is the most widely used coumarin because it has a predictable onset and duration of action and excellent bioavailability.32 Others, such as acenocoumarol (Sintrom) and phenprocoumon (Marcoumar), are used more outside the United States but can be ordered or brought into the United States.
Coumarins interfere with vitamin K metabolism, inhibiting the generation of vitamin-K-dependent procoagulant proteins (factors II, VII, IX, and X) and thereby preventing clotting. They also inhibit the formation of the vitamin-K-dependent intrinsic anticoagulant proteins C and S.
Major bleeding is the most significant side effect of coumarin therapy, occurring at a rate of 4% to 6% over 3 months when the prothrombin time is maintained at an international normalized ratio (INR) of 2 to 3,33 and more often if the INR is higher.
Other issues with warfarin are the effect of variations in dietary vitamin K intake on anticoagulation and potential drug interactions that may alter the anticoagulant effect. Thus, the INR needs to be monitored closely.