Deep brain stimulation: How does it work?
ABSTRACT
Deep brain stimulation has significantly improved the motor symptoms in patients with Parkinson's disease (PD) and other movement disorders. The mechanisms responsible for these improvements continue to be explored. Inhibition at the site of stimulation has been the prevailing explanation for the symptom improvement observed with deep brain stimulation. Research using microelectrode recording during deep brain stimulation in the MPTP monkey model of PD has helped clarify how electrical stimulation of structures within the basal ganglia-thalamocortical circuit improves motor symptoms, and suggests that activation of output and the resultant change in pattern of neuronal activity that permeates throughout the basal ganglia motor circuit is the mechanism responsible for symptom improvement.
Patterns of activity are more important than rate
Knowledge that stimulation activated output from the stimulated region and changed the pattern of neuronal activity led us to ponder whether other targets, or even other ways to deliver stimulation, might work better to improve parkinsonian symptoms.
A focus on GPe stimulation
As a result of these observations, we reasoned that since GPe activity is also altered in PD and its rates are reduced, driving the output from this region that is inhibitory to the STN and GPi may help to reduce and regularize that activity at a point in the circuit that could provide even greater improvement in the motor symptoms associated with PD. Based on this hypothesis, we performed direct stimulation of the GPe in the MPTP monkey model of PD and evaluated its effect on motor behavior and neuronal activity in the circuit.
As an interesting sidelight, it should be noted that long before we developed this hypothesis, we had observations from a 1994 experiment (only recently published20) in which bradykinesia was improved upon acute stimulation in the GPe prior to making a lesion in the GPi. With sustained stimulation in this patient, we observed development of dyskinetic movements. Since we reasoned that lesions in this region would worsen parkinsonian symptoms—a rationale recently supported by a publication from our laboratory in 200619—and since we had no means by which to stimulate this region chronically at the time, this observation was filed away and we continued with lesioning the GPi for the treatment of these patients.
However, with the advent of chronic deep brain stimulation, we opted to reexplore this series of experiments in MPTP-treated monkeys. A lead was placed such that three of its contacts were in the GPe and one was in the GPi. Bradykinesia was assessed by determining the time it took for the monkey to retrieve raisins from a Klüver board. By inducing symptoms on one side only, we were able to use the healthy side as a control. We observed that before stimulation, retrieval took more than twice as long on the affected side. Stimulation of only 2 V had no effect, but increasing the voltage to 5.5 V significantly improved retrieval time.21
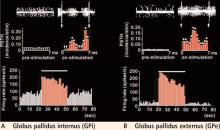
Plotting the data using post-stimulus time histograms showed that stimulation of the GPe inhibited the STN, confirming our hypothesis that stimulation activated the output from the stimulated structure (the GPe sends inhibitory projections to the STN). The responses observed were dramatic, with the majority of cells in the STN showing almost complete suppression of activity (Vitek et al, unpublished data).
In light of this observation, we expected that the rate of activity in the GPi would be reduced. Interestingly, although the rate was changed in most cells compared with control, what was most striking was the relatively stereotyped pattern of inhibition and excitation that occurred following each pulse of GPe stimulation. Although shifted in absolute frequency, the pattern that occurred was similar to that observed during STN stimulation, with alternating periods of excitation and inhibition evident in the post-stimulus time histogram.
Further evaluation of the data revealed a change in burst and oscillatory activity in the STN. Analysis of the data showed a shift in the distribution of power from low to high frequencies. Stimulation reduced activity in the low-frequency range and increased power in higher frequencies, similar to that in normal movement.
Further analysis of the spike trains revealed that entropy (a reflection of noise in the spike signal) was reduced under stimulation parameters that resulted in a reduction in symptoms. In contrast, stimulation parameters that resulted in worsening symptoms increased measures of entropy (Dorval, data submitted for publication).
PATTERN CHANGES AFFECT INFORMATION PROCESSING ACROSS THE BASAL GANGLIA–THALAMOCORTICAL NETWORK
There is a lack of consensus about the precise physiologic effect of deep brain stimulation for improving symptoms in movement disorders. Many researchers continue to believe that deep brain stimulation works through inhibition. An alternate explanation is that at effective stimulation parameters, the net effect is activation of output from the stimulated structure. Various modalities, including modeling,22,23 microdialysis,24 functional magnetic resonance imaging,25 and positron emission tomography,26,27 provide additional evidence that activation occurs during stimulation.
While one cannot discount a role for rate changes in mediating the effects of deep brain stimulation, there is now increasing evidence suggesting that pattern changes induced in the network as a result of stimulation-induced activation of output from the stimulated structure play an integral role in this process.
Research often leads to unpredictable outcomes. The prevailing hypothesis a decade ago concerning the pathophysiologic basis of PD (and still believed in many centers) was that rate is the controlling factor. But we have seen in our animal models that symptoms improve with increased rate in the GPi during stimulation in the STN. Similarly, GPi rates are abnormally low in patients with dystonia and in PD patients during dyskinesia, yet lesioning in the GPi that further reduces its output leads to improvement in these conditions. Based on these observations, it would appear that rate is unlikely to be the critical factor; we now must take into account other factors, such as pattern, oscillation, and synchronization, as well as changes in the network dynamics. Deep brain stimulation is changing the informational content of the neural network, and these changes are occurring across populations of neurons through the whole basal ganglia circuit. Knowing how these changes result in improvement in the neurologic disorder being treated will be critical to our understanding of not only how deep brain stimulation works, but how to make it work better and how to apply it effectively to other neurologic disorders.
FUTURE DIRECTIONS
Future research should focus on multiunit recording simultaneously across nodal points in the basal ganglia–thalamocortical circuit to assess population and network dynamics. This approach would provide information on the real-time effects of stimulation in the network. Until now, most studies have collected recordings from one cell at a time. This is a very labor-intensive process and limits our ability to relate what happens at one point in the circuit to what happens at another point. Multiunit recording across multiple nodes within the basal ganglia–thalamocortical circuit will help us address this question and tell us what happens across populations of neurons at multiple sites in the motor circuit and how this is changed during stimulation. Such an approach will help us to better understand the pathophysiologic basis for the development of neurologic disorders and how stimulation works to improve these disorders. This information is a critical step toward the ability to knowingly change network activity in a way that is predictable and more compatible with the normal state, as well as toward the application of this technology to other disorders.
The potential for clinical applications of deep brain stimulation is dramatic, but we must proceed with caution. Indications should be based on sound scientific rationale, and outcomes must be accurately and systematically documented. Move forward we must, but with caution—most certainly.
Acknowledgments
The author thanks Dr. Jianyu Zhang for his work in preparing Figure 2 and Drs. Svjetlana Miocinovic and Cameron McIntyre for their work in developing the software program Cicerone that was used to prepare this figure. The author also thanks Drs. Takao Hashimoto, Jianyu Zhang, and Weidong Xu for their vital contributions to our deep brain stimulation research program, without which none of this work would have been possible.