Monitoring pulmonary complications in long-term childhood cancer survivors: Guidelines for the primary care physician
ABSTRACTCurative therapy for childhood cancers poses the risk of long-term complications, necessitating regular lifelong follow-up for survivors. The Children’s Oncology Group (COG) has issued guidelines on this topic (www.survivorshipguidelines.org). This review summarizes the findings of the COG Guideline Task Force on Pulmonary Complications with respect to pulmonary toxicity.
KEY POINTS
- Radiation therapy causes pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung disease. The risk is dose-dependent and increases with concomitant chemotherapy, younger age at treatment, atopic history, and smoking.
- Alkylating agents cause pulmonary fibrosis. Bleomycin can cause interstitial pneumonitis, pulmonary fibrosis, or, very rarely, acute respiratory distress syndrome.
- Cancer survivors should have a yearly history and physical examination, plus pulmonary function testing and radiography at baseline and repeated as clinically indicated.
- All patients who smoke should be encouraged to quit.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
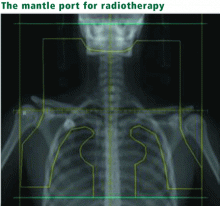
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.