Monitoring pulmonary complications in long-term childhood cancer survivors: Guidelines for the primary care physician
ABSTRACTCurative therapy for childhood cancers poses the risk of long-term complications, necessitating regular lifelong follow-up for survivors. The Children’s Oncology Group (COG) has issued guidelines on this topic (www.survivorshipguidelines.org). This review summarizes the findings of the COG Guideline Task Force on Pulmonary Complications with respect to pulmonary toxicity.
KEY POINTS
- Radiation therapy causes pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung disease. The risk is dose-dependent and increases with concomitant chemotherapy, younger age at treatment, atopic history, and smoking.
- Alkylating agents cause pulmonary fibrosis. Bleomycin can cause interstitial pneumonitis, pulmonary fibrosis, or, very rarely, acute respiratory distress syndrome.
- Cancer survivors should have a yearly history and physical examination, plus pulmonary function testing and radiography at baseline and repeated as clinically indicated.
- All patients who smoke should be encouraged to quit.
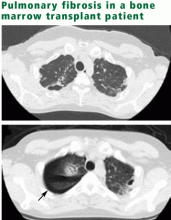
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.