Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI
ABSTRACTNephrogenic systemic fibrosis (NSF) is a newly recognized systemic disorder characterized by widespread tissue fibrosis in patients with impaired renal function. Recent reports suggest that NSF is associated with exposure to gadolinium-based contrast agents used in magnetic resonance imaging. NSF can be very debilitating and can lead to serious complications and death. Health care providers should exercise caution when considering the use of gadolinium-based imaging studies in patients with renal dysfunction.
KEY POINTS
- NSF seems to arise in roughly 3% of patients with renal insufficiency who receive gadolinium, although the data are somewhat sketchy and the true incidence might be higher if the NSF is specifically looked for.
- Manufacturers of all available gadolinium contrast agents now must include a boxed warning about the risk of NSF in patients with acute or chronic severe renal insufficiency (glomerular filtration rate < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
- As yet, we have no effective treatment for NSF. If the patient is already on hemodialysis, it may be reasonable to perform hemodialysis immediately after exposure to gadolinium and again the next day.
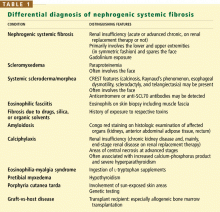
DIFFERENTIAL DIAGNOSIS
An important diagnostic feature of NSF is that it spares the face, a finding derived from all reported and confirmed cases of NSF (Figure 2). In contrast, scleromyxedema, systemic scleroderma, and morphea often involve the face.
Scleromyxedema is often associated with monoclonal gammopathy (usually an immunoglobulin G lambda paraproteinemia) whereas NSF is not.
Scleroderma is supported by the findings of Raynaud’s phenomenon, antinuclear antibodies, and either anticentromere or anti-DNA topoisomerase I (Scl-70) antibodies, but the absence of these antibodies does not necessarily rule it out.
Eosinophilic fasciitis is diagnosed on the basis of histologic examination of a deep wedge skin biopsy specimen that includes fascia.
Other diagnoses that should be considered include amyloidosis and calciphylaxis.
ASSOCIATION WITH GADOLINIUM: WHAT IS THE EVIDENCE?
Case series
The association of gadolinium use with NSF has been described in several case reports and case series.
Grobner11 reported that administration of gadodiamide (Omniscan, a gadolinium compound) for MRI was associated with NSF in five patients on chronic hemodialysis who had end-stage renal disease. Their ages ranged from 43 to 74 years, and they had been on dialysis from 10 to 58 months. The time of onset of NSF ranged from 2 to 4 weeks after exposure to gadodiamide.
Marckmann et al12 reported that NSF developed in 13 (3.5%) of 370 patients with severe kidney disease who received gadodiamide. Five of the 13 patients had stage 5 (advanced) chronic kidney disease and were not yet on renal replacement therapy, 7 were on hemodialysis, and 1 was on peritoneal dialysis. The time of onset ranged from 2 to 75 days (median 25 days) after exposure.
Kuo et al13 similarly estimated the incidence of NSF at approximately 3% in patients with severe renal failure who receive intravenous gadolinium-based contrast material for MRI.
Broome et al14 reported that 12 patients developed NSF within 2 to 11 weeks after receiving gadodiamide. Eight of the 12 patients had end-stage renal disease and were on hemodialysis; the other 4 patients had acute kidney injury attributed to hepatorenal syndrome, and 3 of these 4 patients were on hemodialysis.
Khurana et al15 reported that 6 patients on hemodialysis developed NSF from 2 weeks to 2 months after receiving a dose of gadodiamide of between 0.11 and 0.36 mmol/kg. These doses are high, and the findings suggest an association between the gadolinium dose and NSF. The dose approved by the US Food and Drug Administration (FDA) is only 0.1 mmol/kg, and the use of gadolinium is approved only in MRI. However, higher doses (0.3–0.4 mmol/kg) are widely used in practice for better imaging quality in magnetic resonance angiography (MRA).
Deo et al16 reported 3 cases of NSF in 87 patients with end-stage renal disease who underwent 123 radiologic studies with gadolinium. No patient with end-stage renal disease who was not exposed to gadolinium developed NSF, and the association between exposure to gadolinium and the subsequent development of NSF was statistically significant (P = .006). The authors concluded that each gadolinium study presented a 2.4% risk of NSF in end-stage renal disease patients.
This retrospective study is flawed by not having been cross-sectional or case-controlled, since the other 84 patients who received gadolinium were not examined at all to establish the absence of NSF.
Case-control studies
More evidence of association of NSF with gadolinium exposure comes from other reports.
Physicians in St. Louis, MO,17 identified 33 cases of NSF and performed a case-control study, matching each of 19 of the patients (for whom data were available and who met their entry criteria) with 3 controls. They found that exposure to gadolinium was independently associated with the development of NSF.
Sadowski et al18 reported that 13 patients with biopsy-confirmed NSF all had been exposed to gadodiamide and one had been exposed to gadobenate (MultiHANCE) in addition to gadodiamide. All 13 patients had renal insufficiency, with an estimated glomerular filtration rate (GFR) less than 60 mL/minute/1.73 m2. The investigators compared this group with a control group of patients with renal insufficiency who did not develop NSF. The NSF group had more proinflammatory events (P < .001) and more gadolinium-contrast-enhanced MRI examinations per patient (P = .002) than the control group.
Marckmann et al19 compared 19 patients who had histologically proven cases of NSF and 19 sex- and age-matched controls; all 38 patients had chronic kidney disease and had been exposed to gadolinium. Patients with NSF had received higher cumulative doses of gadodiamide and higher doses of erythropoietin and had higher serum concentrations of ionized calcium and phosphate than did their controls, as did patients with severe NSF compared with those with nonsevere NSF.
Comment. All the above reports are limited by their study design and suffer from recognition bias because not all of the patients with severe renal insufficiency who were exposed to gadolinium were examined for possible asymptomatic skin changes that might be characteristic of NSF. Therefore, it is impossible to be certain that all of the patients classified as not having NSF truly did not have it or did not subsequently develop it. Furthermore, the reports lacked standardized diagnostic criteria. Hence, the real prevalence and incidence of NSF are difficult to determine.