The rationale for, and design of, a lung cancer screening program
ABSTRACTWe are entering a new era in which lung cancer screening may be considered the standard of care. The National Lung Screening Trial (NLST) has shown that the number of deaths due to lung cancer can be reduced through screening with low-dose computed tomography (CT) in a high-risk population (N Engl J Med 2011; 365:395–409). Key issues—such as how to manage lung nodules, how to improve cost-effectiveness, and how to minimize radiation exposure—need to be addressed when designing a lung cancer screening program. Time and further technical advances will help to optimize the programs that are developed.
KEY POINTS
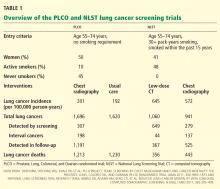
- The NLST documented a 20% reduction in the rate of death from lung cancer with low-dose CT screening compared with chest radiography screening (number needed to treat = 320). This was in a population at high risk (age 55–74 with a smoking history of at least 30 pack-years, at least some of it within the past 15 years).
- CT screening detects many lung nodules, of which only a few (3.6% in the NLST) prove to be cancer.
- In view of the positive results of the NLST, Cleveland Clinic has begun a lung cancer screening program, using the same entry criteria as those in the NLST.
- Of possibly greater impact than detecting lung cancer will be the opportunity to promote smoking cessation.
In 2011, two papers were published that will shape the way we think about lung cancer screening for years to come.
See related patient information sheet
While the ability to screen for lung cancer is a major positive change, it also raises many thorny questions, such as who should be screened, how often should they be screened, and how should we respond when a nodule is detected.
To answer some of these questions, we will outline how Cleveland Clinic has structured its lung cancer screening program, and the rationale we used for making pragmatic patient-care decisions within this program. We will conclude with our thoughts about the potential evolution of lung cancer screening programs.
THE 40-YEAR QUEST FOR EFFECTIVE LUNG CANCER SCREENING
Lung cancer kills more people in the United States than the next four most lethal types of cancer combined.3 It is curable if found early in its course. Unfortunately, most people who develop lung cancer feel no symptoms when it is early in its course, and therefore it is too often diagnosed at a late stage. Treatment for late-stage lung cancer is effective, but it is rarely curative.
Screening refers to testing people at risk of developing a disease before its symptoms or signs have appeared. The goal of screening is to reduce the disease-specific mortality rate. For this to happen, the disease must be detectable in a preclinical form, and treatment must be more successful when applied early. Ideally, the screening test should pose little risk to the patient, be sensitive for detecting the disease early in its course, give few false-positive results, be acceptable to the patient, and be relatively inexpensive to the health system.
Over the past 4 decades, a large volume of research has been done in the hope of proving that conventional radiography or CT could be an effective screening test for lung cancer.4,5
Cohort studies (ie, in which all the patients were screened) of radiography or CT have shown a longer survival from the time of lung cancer diagnosis than would be expected without screening. These studies were not designed to prove a reduction in the lung cancer-specific mortality rate.
Controlled trials (in which half the patients received the screening and the other half did not) of chest radiography have been interpreted as not showing a reduction in lung cancer mortality rates, though debate about the interpretation of these trials persisted until this past year. Biases inherent in using duration of survival rather than the mortality rate as an end point have been suggested as the reason for the apparent benefit in survival without a reduction in the mortality rate.
Controlled trials of CT screening were started nearly a decade ago. Until 2011, the results of these trials were not mature enough to comment on.
THE PROSTATE, LUNG, COLORECTAL, AND OVARIAN TRIAL
The lung cancer screening portion of the PLCO trial aimed to determine the effect of screening chest radiography on lung cancer-specific mortality rates.1
In this trial, 154,901 people were randomized to undergo either posteroanterior chest radiography every year for 4 years or usual care, ie, no lung cancer screening. Participants were men and women age 55 to 74 with no history of prostate, lung, colorectal, or ovarian cancer. They did not need to be a smoker to participate. Those who had never smoked and who were randomized to the screening group received only 3 years of testing. All were followed for 13 years or until the conclusion of the study (8 years after the final participant was enrolled). About half were women, and nearly two-thirds were age 55 through 64. Only 10% were current smokers, while a full 45% had never smoked.
Results. Adherence to screening in the screening group ranged from 79% to 86.6% over the years of screening, and 11% of the usual-care group was estimated to have undergone screening chest radiography.
Cumulative lung cancer incidence rates were 201 per 100,000 person-years in the screening group and 192 in the usual-care group.
In the screening group, there were a total of 1,696 lung cancers during the entire study. Of these, 307 (18%) were detected by screening, 198 (12%) were interval cancers (diagnosed during the screening period but not by the screening test), and the remainder were diagnosed after the screening period during the years of follow-up. In the screening group, the cancers detected by screening were more likely to be adenocarcinomas and less likely to be small-cell carcinomas than those not detected by screening. Also in the screening group, the cancers detected by screening were more likely to be stage I (50%) than those not detected by screening.
The cumulative number of deaths from lung cancer was slightly but not significantly lower in the screening group from years 4 through 11. However, by the end of follow-up, the number of lung cancer deaths was equal between the groups (1,213 in the screening group vs 1,230 in the usual-care group). The cumulative overall mortality rate was also similar between the groups. For the subgroup who would have qualified for the NLST (see below), the lung cancer mortality rate was statistically similar between the two groups.
Comments. The results of the PLCO screening trial will be interpreted as the final word in lung cancer screening with standard chest radiography. The conclusion is that annual screening with chest radiography does not reduce lung cancer mortality rates and thus should not be performed in this context.