Beyond depression: Other uses for tricyclic antidepressants
ABSTRACT
Tricyclic antidepressants (TCAs) were originally designed and marketed for treating depression, but over time they have been applied to a variety of conditions, mostly off-label. TCAs can serve as first-line or augmenting drugs for neuropathic pain, headache, migraine, gastrointestinal syndromes, fibromyalgia, pelvic pain, insomnia, and psychiatric conditions other than depression. This article reviews pharmacology, dosing, and safety considerations for these uses.
KEY POINTS
- Amitriptyline is the most useful TCA for many painful conditions.
- TCAs can be especially helpful for patients with a pain syndrome or insomnia with comorbid depression, although their benefits appear to be independent of antidepressant effects.
- TCAs have long half-lives and so can be taken once a day.
- Effective dosages for symptom control in many conditions are lower than for severe depression; dosage should start low and be gradually increased while monitoring efficacy and adverse effects.
- TCAs should not be used concurrently with a monoamine oxidase inhibitor and by certain patient groups: the elderly, pregnant women, and patients with certain cardiac conduction abnormalities, epilepsy, or risk of suicide.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
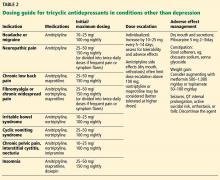
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.