Beyond depression: Other uses for tricyclic antidepressants
ABSTRACT
Tricyclic antidepressants (TCAs) were originally designed and marketed for treating depression, but over time they have been applied to a variety of conditions, mostly off-label. TCAs can serve as first-line or augmenting drugs for neuropathic pain, headache, migraine, gastrointestinal syndromes, fibromyalgia, pelvic pain, insomnia, and psychiatric conditions other than depression. This article reviews pharmacology, dosing, and safety considerations for these uses.
KEY POINTS
- Amitriptyline is the most useful TCA for many painful conditions.
- TCAs can be especially helpful for patients with a pain syndrome or insomnia with comorbid depression, although their benefits appear to be independent of antidepressant effects.
- TCAs have long half-lives and so can be taken once a day.
- Effective dosages for symptom control in many conditions are lower than for severe depression; dosage should start low and be gradually increased while monitoring efficacy and adverse effects.
- TCAs should not be used concurrently with a monoamine oxidase inhibitor and by certain patient groups: the elderly, pregnant women, and patients with certain cardiac conduction abnormalities, epilepsy, or risk of suicide.
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
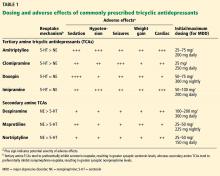
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.