Current management of Barrett esophagus and esophageal adenocarcinoma
Release date: November 1, 2019
Expiration date: October 31, 2020
Estimated time of completion: 1 hour
Click here to start this CME/MOC activity.
ABSTRACT
Barrett esophagus is found in 5% to 15% of patients with gastroesophageal reflux disease and is a precursor of esophageal adenocarcinoma, yet the condition often goes undiagnosed. Patients with reflux disease who are male, over age 50, or white, and who smoke or have central obesity or a family history of Barrett esophagus or esophageal adenocarcinoma, should undergo initial screening endoscopy and, if no dysplasia is noted, surveillance endoscopy every 3 to 5 years. Dysplasia is treated with endoscopic eradication by ablation, resection, or both. Chemoprotective agents are being studied to prevent progression to dysplasia in Barrett esophagus. The authors discuss current recommendations for screening and management.
KEY POINTS
- Screening is recommended for patients with long-standing reflux symptoms (> 5 years) and 1 or more key risk factors: male sex, age over 50, white race, central obesity, and history of smoking.
- In Barrett esophagus without dysplasia, surveillance endoscopy is recommended every 3 to 5 years to detect dysplasia and early esophageal adenocarcinoma.
- The recommended treatment of dysplasia is endoscopic eradication followed by surveillance endoscopy.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
,Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
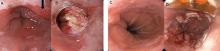
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41