Current management of Barrett esophagus and esophageal adenocarcinoma
Release date: November 1, 2019
Expiration date: October 31, 2020
Estimated time of completion: 1 hour
Click here to start this CME/MOC activity.
ABSTRACT
Barrett esophagus is found in 5% to 15% of patients with gastroesophageal reflux disease and is a precursor of esophageal adenocarcinoma, yet the condition often goes undiagnosed. Patients with reflux disease who are male, over age 50, or white, and who smoke or have central obesity or a family history of Barrett esophagus or esophageal adenocarcinoma, should undergo initial screening endoscopy and, if no dysplasia is noted, surveillance endoscopy every 3 to 5 years. Dysplasia is treated with endoscopic eradication by ablation, resection, or both. Chemoprotective agents are being studied to prevent progression to dysplasia in Barrett esophagus. The authors discuss current recommendations for screening and management.
KEY POINTS
- Screening is recommended for patients with long-standing reflux symptoms (> 5 years) and 1 or more key risk factors: male sex, age over 50, white race, central obesity, and history of smoking.
- In Barrett esophagus without dysplasia, surveillance endoscopy is recommended every 3 to 5 years to detect dysplasia and early esophageal adenocarcinoma.
- The recommended treatment of dysplasia is endoscopic eradication followed by surveillance endoscopy.
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
,The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
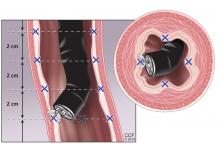
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21