Monoclonal gammopathy of undetermined significance: A primary care guide
Release date: January 1, 2019
Expiration date: December 31, 2019
Estimated time of completion: 1 hour
Click here to start this CME/MOC activity.
ABSTRACT
Monoclonal gammopathy of undetermined significance (MGUS) is commonly diagnosed in outpatients being worked up for an array of clinical concerns. It carries a risk of progression to myeloma and other lymphoproliferative disorders that, albeit low (1% per year), warrants regular follow-up. Patients with MGUS can be risk-stratified on the basis of the amount and type of their monoclonal protein as well as whether they have an abnormal light-chain ratio. Here, we provide a guide to the diagnosis, workup, and management of MGUS.
KEY POINTS
- MGUS is the most common of the monoclonal gammopathies.
- The overall risk of MGUS progressing to myeloma and other lymphoproliferative disorders is 1% per year.
- Low-risk MGUS is defined by an immunoglobulin G monoclonal protein at a concentration less than 1.5 g/dL and a normal serum free light-chain ratio.
- Low-risk MGUS carries a much lower risk of progression than intermediate- and high-risk MGUS, may not require subspecialty referral, and can be followed by the outpatient provider.
HOW ARE PATIENTS WITH MGUS RISK-STRATIFIED AND FOLLOWED?
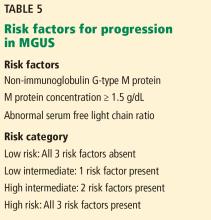
Research has helped to refine the diagnostic workup and recognize subsets of patients with MGUS at different risks of progression to myeloma and related disorders. Factors predicting progression are 1,6,7,26,27:
- The amount of the M protein
- The type of M protein (IgG vs non-IgG)
- An abnormal free light-chain ratio.
Half of patients with MGUS fall into the low-risk category, which is defined by IgG-type serum M protein in a concentration less than 1.5 g/dL and a normal serum free light-chain ratio (kappa-lambda 0.26–1.65).5,27 The absolute risk of progression at 20 years is only 5% for patients with low-risk MGUS, compared with 58% in patients with high-risk MGUS (positive for all 3 risk factors).5
,The presence of less than 10% plasma cells in the bone marrow is required to satisfy the definition of MGUS, but bone marrow biopsy can be omitted for patients with low-risk MGUS, given the slim chance of finding a significant percentage of clonal plasma cells in the marrow and the inherently low risk of progression.5,10 Skeletal surveys are often deferred for low-risk MGUS, but we obtain them in all our patients to ensure the absence of plasmacytomas, which need to be treated (typically with radiotherapy). Importantly, patients with unexplained bone pain (mostly in long bones, ribs, and spine, whereas joints are not typically involved) and a normal skeletal survey should undergo advanced imaging (whole-body magnetic resonance imaging or whole-body positron emission tomography and computed tomography) to detect bone lesions otherwise missed on plain radiography.28,29
Most of the recommendations regarding follow-up are based on expert opinion, given the lack of randomized data. Most experts agree that all patients should be reevaluated 6 months after an M protein is detected, with laboratory surveillance tests (complete blood cell count, serum creatinine, serum calcium level, serum protein electrophoresis, and serum free light chains). Low-risk patients with a stable M protein level can be followed every 2 to 3 years.
Suspect malignant progression if the serum M protein level increases by 50% or more (with an absolute increase of ≥ 0.5 g/dL); the serum M protein level is 3 g/dL or higher; the serum free light-chain ratio is more than 100; or the patient has unexplained anemia, elevated creatinine, bone pain, fracture, or hypercalcemia.
Patients at intermediate or high risk should be followed annually after the initial 6-month visit.5,7,10
A recent study highlighted the importance of risk stratification in reducing the costs associated with an overzealous diagnostic workup of patients with low-risk MGUS.30 These savings are in addition to a reduction in patient anticipation and anxiety that universally occur before invasive procedures.
THE ROLE OF THE PRIMARY CARE PROVIDER AND THE HEMATOLOGIST
Once an M protein is identified, a comprehensive history, physical examination, and laboratory tests (serum protein electrophoresis to quantify the protein, serum immunofixation, serum free light chains, complete blood cell count, calcium, and creatinine) should be done, taking into consideration the differential diagnosis of monoclonal gammopathies discussed above. After MGUS is confirmed, the patient should be risk-stratified to determine the need for bone marrow biopsy and to predict the risk of progression to more serious conditions.
Referral to a hematologist is warranted for patients with intermediate- and high-risk MGUS, patients with abnormal serum free light-chain ratios, and those who show evidence of malignant progression. Patients with intermediate- and high-risk MGUS could be referred for bone marrow biopsy before assessment by a hematologist. The primary care provider may continue to follow patients with low-risk MGUS who do not display clinical or laboratory evidence of myeloma or related disorders.
The importance of educating patients to report any new worrisome symptom (eg, fatigue, neuropathy, weight loss, night sweats, bone pain) cannot be overemphasized, as some patients may progress to myeloma or other disorders between follow-up visits.