Monoclonal gammopathy of undetermined significance: A primary care guide
Release date: January 1, 2019
Expiration date: December 31, 2019
Estimated time of completion: 1 hour
Click here to start this CME/MOC activity.
ABSTRACT
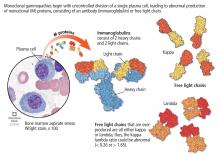
Monoclonal gammopathy of undetermined significance (MGUS) is commonly diagnosed in outpatients being worked up for an array of clinical concerns. It carries a risk of progression to myeloma and other lymphoproliferative disorders that, albeit low (1% per year), warrants regular follow-up. Patients with MGUS can be risk-stratified on the basis of the amount and type of their monoclonal protein as well as whether they have an abnormal light-chain ratio. Here, we provide a guide to the diagnosis, workup, and management of MGUS.
KEY POINTS
- MGUS is the most common of the monoclonal gammopathies.
- The overall risk of MGUS progressing to myeloma and other lymphoproliferative disorders is 1% per year.
- Low-risk MGUS is defined by an immunoglobulin G monoclonal protein at a concentration less than 1.5 g/dL and a normal serum free light-chain ratio.
- Low-risk MGUS carries a much lower risk of progression than intermediate- and high-risk MGUS, may not require subspecialty referral, and can be followed by the outpatient provider.
MGUS is present in 3% to 4% of the population over age 50 and is more common in older men, African Americans, and Africans.1–6
The overall risk of progression to myeloma and related disorders is less than or equal to 1% per year depending on the subtype of the M protein (higher risk with IgM than non-IgM and light-chain MGUS).7,8 While the risk of malignant transformation is low, multiple myeloma is almost always preceded by the presence of an asymptomatic and often unrecognized monoclonal protein.
,WHEN SHOULD WE LOOK FOR AN M PROTEIN?
An M protein is typically an incidental finding when a patient is being assessed for any of a number of presenting symptoms or conditions. A large retrospective study9 found that screening for MGUS was mostly performed by internal medicine physicians. The indications for testing were anemia, bone-related issues, elevated creatinine, elevated erythrocyte sedimentation rate, and neuropathy.
A low anion gap is not a major indicator of an M protein unless in a high concentration, in which case other manifestations would be present, such as renal failure, which would guide the diagnosis. Polyclonal hypergammaglobulinemia as a cause of low anion gap is far more common than MGUS.
HOW SHOULD WE SCREEN FOR AN M PROTEIN?
Serum protein electrophoresis is an initial test used to identify an M protein and has a key role in quantifying it (Figure 2). An M protein appears as a narrow spike on the agarose gel and should be distinguished from the broad band seen in polyclonal gammopathies associated with cirrhosis and chronic infectious and inflammatory conditions, among others.12 A major disadvantage of serum protein electrophoresis is that it cannot detect an M protein in very low concentrations or determine its identity.
Serum immunofixation is more sensitive than serum protein electrophoresis and should always be ordered in conjunction with it, mostly to ensure detecting tiny amounts of M protein and to identify the type of its heavy chain and light-chain components.13
The serum free light-chain assay is also considered an essential part of the screening process to detect light-chain MGUS and light-chain myeloma. As many as 16% of myeloma patients secrete only light chains, which may not be identified on serum immunofixation.3,6,7,10,14,15 In general, a low kappa-lambda ratio (< 0.26) indicates the overproduction of lambda light chains, and a high ratio (> 1.65) indicates the overproduction of kappa light chains.
The serum free light-chain assay helps detect abnormal secretion of monoclonal light chains before they appear in the urine once the kidney tubules become saturated and unable to reabsorb them.
Of note, the free light-chain ratio can be abnormal (< 0.26 or > 1.65) in chronic kidney disease. Thus, it may be challenging to discern whether an abnormal light-chain ratio is related to impaired light-chain clearance by the kidneys or to MGUS. In general, kappa light chains are more elevated than lambda light chains in chronic kidney disease, but the ratio should not be considerably skewed. A kappa-lambda ratio below 0.37 or above 3 is rarely seen in chronic kidney disease and should prompt workup for MGUS.16
Tests in combination. The sensitivity of screening for M proteins ranges from 82% with serum protein electrophoresis alone to 93% with the addition of serum immunofixation and to 98% with the serum free light-chain assay.15 The latter can replace urine protein electrophoresis and immunofixation when screening for M protein, given its higher sensitivity.15,17 An important caveat is that urine dipstick testing does not detect urine light chains.
Table 3 lists the initial laboratory tests required in patients with MGUS.