Beta-cell therapies for type 1 diabetes: Transplants and bionics
ABSTRACT
Research continues toward the goal of treating type 1 diabetes by replacing insulin-producing beta cells. Ideally, such treatment would be safe and long-lasting and would eliminate the need for subcutaneous insulin replacement. This article reviews the current state of beta-cell replacement through transplant of the whole pancreas or of islet cells. It also looks at the “bionic” pancreas and other future challenges.
KEY POINTS
- Most pancreas transplant recipients become insulin-independent immediately.
- A key drawback to islet transplant is the need for multiple donors to provide enough islet cells to achieve insulin independence.
- As with other organs for transplant, the need for donor pancreases far outnumbers the supply. Stem cells or beta cells grown from stem cells may avoid this problem. Another potential solution is to use organs from animals, possibly pigs, but much more work is needed to make these procedures viable.
- While we await a breakthrough in beta-cell therapy, a bionic pancreas may be the answer for a number of patients.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
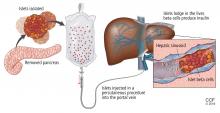
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.