Beta-cell therapies for type 1 diabetes: Transplants and bionics
ABSTRACT
Research continues toward the goal of treating type 1 diabetes by replacing insulin-producing beta cells. Ideally, such treatment would be safe and long-lasting and would eliminate the need for subcutaneous insulin replacement. This article reviews the current state of beta-cell replacement through transplant of the whole pancreas or of islet cells. It also looks at the “bionic” pancreas and other future challenges.
KEY POINTS
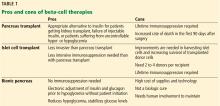
- Most pancreas transplant recipients become insulin-independent immediately.
- A key drawback to islet transplant is the need for multiple donors to provide enough islet cells to achieve insulin independence.
- As with other organs for transplant, the need for donor pancreases far outnumbers the supply. Stem cells or beta cells grown from stem cells may avoid this problem. Another potential solution is to use organs from animals, possibly pigs, but much more work is needed to make these procedures viable.
- While we await a breakthrough in beta-cell therapy, a bionic pancreas may be the answer for a number of patients.
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.