Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus
ABSTRACT
Nutrient intake stimulates the secretion of the gastrointestinal incretin hormones, glucagon-like peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which exert glucose-dependent insulinotropic effects and assist pancreatic insulin and glucagon in maintaining glucose homeostasis. GLP-1 also suppresses glucose-dependent glucagon secretion, slows gastric emptying, increases satiety, and reduces food intake. An impaired incretin system, characterized by decreased responsiveness to GIP and markedly reduced GLP-1 concentration, occurs in individuals with type 2 diabetes mellitus (T2DM). The administration of GLP-1 improves glycemic control, but GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase–4 (DPP-4). Exenatide, a DPP-4–resistant exendin-4 GLP-1 receptor agonist, exhibits the glucoregulatory actions of GLP-1 and reduces body weight in patients with T2DM. It may possess cardiometabolic actions with the potential to improve the cardiovascular risk profile of patients with T2DM. DPP-4 inhibitors such as sitagliptin and saxagliptin increase endogenous GLP-1 concentration and demonstrate incretin-associated glucoregulatory actions in patients with T2DM. DPP-4 inhibitors are weight neutral. A growing understanding of the roles of incretin hormones in T2DM may further clarify the application of incretin-based treatment strategies.
KEY POINTS
- The incretin effect may be responsible for up to 70% of insulin secretion following oral glucose ingestion; reduction of the incretin effect contributes to T2DM pathophysiology.
- It is unknown whether incretin defects are a cause or consequence of T2DM.
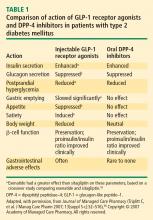
- Incretin therapies effectively lower glucose with concomitant favorable effects on body weight. GLP-1 receptor agonists reduce weight, while DPP-4 inhibitors are weight neutral.
TARGETING FUNDAMENTAL DEFECTS OF T2DM WITH INCRETIN-BASED THERAPIES
Recognition and a better understanding of the role of the incretins and the enzyme involved in their degradation have led to the development of two incretin-based treatments: the GLP-1 receptor agonists, which possess many of the glucoregulatory actions of incretin peptides, and the DPP-4 inhibitors.5 Both the GLP-1 receptor agonists and the DPP-4 inhibitors have demonstrated safety and efficacy in the management of hyperglycemia in patients with T2DM.
GLP-1 receptor agonists
The GLP-1 receptor agonist exenatide is a synthetic form of exendin-4 and has a unique amino acid sequence that renders it resistant to degradation by DPP-4, making its actions longer lasting than endogenous GLP-1.5,30 Exenatide has a half-life of 2.4 hours and is detectable for up to 10 hours after subcutaneous (SC) injection.5,30 It is administered BID and has been approved as monotherapy or an adjunct therapy in patients with T2DM who have inadequate glycemic control following treatment with metformin, a sulfonylurea, a thiazolidinedione (TZD), or metformin in combination with a sulfonylurea or a TZD.31–35
In both human and animal studies, exenatide has been shown to enhance glucose-dependent insulin secretion and suppress inappropriate glucagon secretion in a glucose-dependent manner, reduce food intake and body weight, and acutely improve beta-cell function by enhancing first- and second-phase insulin secretion.5,36,37
In a small study involving 17 patients with T2DM, exenatide was shown to slow gastric emptying, which could be an important mechanism contributing to its beneficial effects on PPG concentration.38 Exenatide also has been shown to attenuate postprandial hyperglycemia, a risk factor for cardiovascular disease (CVD), by reducing endogenous glucose production by about 50% in patients with T2DM.39 Another mechanism for glycemic control may exist, as a recent animal study has shown that exenatide, similar to endogenous GLP-1, lowers blood glucose concentration independent of changes in pancreatic islet hormone secretion or delayed gastric emptying.40
A formulation of exenatide that is administered once weekly—exenatide long-acting release (LAR)—is in clinical evaluation and under review by the US Food and Drug Administration (FDA). In a short-term study, exenatide-LAR (0.8 mg or 2.0 mg) was administered once weekly for 15 weeks to patients with T2DM whose glycemia was suboptimally controlled with metformin alone or in combination with diet and exercise. Compared with placebo, treatment with exenatide once weekly was associated with markedly reduced HbA1c, FPG, PPG and body weight.41 In a larger, 30-week, phase 3 trial, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide ONce Weekly (DURATION-1), exenatide-LAR 2 mg once weekly was compared with exenatide 10 mg BID in patients with T2DM. Exenatide-LAR once weekly was associated with a significantly greater reduction in HbA1c (–1.9% vs –1.5%, P = .0023), and with a similar low risk of hypoglycemia and reduction in body weight (–3.7 kg vs –3.6 kg, P = .89) compared with the BID formulation.42
Liraglutide, recently approved in the European Union for T2DM and also under regulatory review in the United States, is a DPP-4–resistant human analogue GLP-1 receptor agonist in clinical development that has a 97% homology to native GLP-1.43–45 In contrast to exenatide, the acetylated liraglutide molecule allows binding to serum albumin and provides resistance to DPP-4 degradation, thus prolonging the half-life of liraglutide to approximately 12 hours. Liraglutide is administered SC QD as monotherapy or in combination with other antidiabetes agents such as metformin or sulfonylurea to patients with T2DM.44–47 Liraglutide has been shown to reduce HbA1c, decrease body weight, and lead to a lower incidence of hypoglycemia compared with the sulfonylurea glimepiride.
DPP-4 inhibitors
Sitagliptin is a DPP-4 inhibitor indicated as monotherapy or in combination with metformin or a TZD in patients with T2DM with inadequate glycemic control.48–51 Given orally, sitagliptin does not bind to the GLP-1 receptor agonist and has been shown to inhibit circulating DPP-4 activity by about 80%.52,53 Sitagliptin has been associated with an approximate twofold increase in postprandial GLP-1 plasma concentrations compared with placebo in healthy human subjects and in patients with T2DM.53 Saxagliptin, another potent DPP-4 inhibitor, significantly reduced HbA1c and FPG concentrations in patients with T2DM54 with a neutral effect on weight; it was recently approved by the FDA for treatment of T2DM.55
The DPP-4 inhibitor vildagliptin is currently being used in the European Union and Latin America but has yet to receive regulatory approval in the United States.54 Alogliptin, a novel, high-affinity, high-specificity DPP-4 inhibitor currently in development, provides rapid and sustained DPP-4 inhibition and significantly reduces HbA1c, FPG, and PPG concentrations with no change in body weight in patients with T2DM.56,57