Perioperative management of warfarin and antiplatelet therapy
ABSTRACT
Perioperative management of patients on warfarin or antiplatelet therapy involves assessing and balancing individual risks for thromboembolism and bleeding. Discontinuing anticoagulant and antiplatelet therapy is usually necessary for major surgery but increases the risk of thrombotic events. Bridge therapy, the temporary perioperative substitution of low-molecular-weight heparin or unfractionated heparin in place of warfarin, is an effective means of reducing the risk of thromboembolism but may increase the risk of bleeding. The timing of warfarin withdrawal and timing of the preoperative and postoperative components of bridge therapy are critical to balancing these risks. Perioperative management of antiplatelet therapy requires special care in patients with coronary stents; the timing of surgery relative to stent placement dictates management in these patients.
KEY POINTS
- Determining when and how to use bridge anticoagulation therapy depends on the patient’s risk for thromboembolism, which is in turn based on the indication for warfarin—ie, a mechanical heart valve, atrial fibrillation, or prior venous thromboembolism.
- Factor patient preference into whether and how to use bridge therapy: many patients are more concerned about stroke risk than bleeding risk, regardless of the relative frequency of these events.
- Anticoagulation with warfarin often does not need to be interrupted for patients undergoing minor surgery, such as some ophthalmic, dental, dermatologic, and gastrointestinal procedures.
- Premature discontinuation of antiplatelet therapy in surgical patients with recent coronary stent placement significantly raises the risk of catastrophic perioperative stent thrombosis.
Perioperative management of surgical patients who require temporary discontinuation of vitamin K antagonists (warfarin) or antiplatelet drugs is complicated. The risk of a thrombotic event during interruption of anticoagulant or antiplatelet therapy must be weighed against the risk of bleeding when such therapy is used in close proximity to a surgical procedure. This balancing of risks is guided by the patient’s individual risk for thromboembolism or bleeding and underlying conditions such as the presence of a mechanical heart valve or a coronary stent.
High-profile adverse events have made anticoagulant and antiplatelet management one of the most highly litigated aspects of perioperative medicine. Moreover, there is a paucity of randomized clinical trial data and definitive guidelines to address the perioperative needs of patients on antithrombotic therapy. Treatment protocols vary depending on many underlying factors, such as the presence of mechanical heart valves, comorbidities, stent type and location, patient age and medical history, and type of surgical procedure. While recent attention has focused on genetic variations that result in higher or lower sensitivity to warfarin in some patients, routine genetic testing for warfarin sensitivity is controversial and not part of widespread practice at this time.
The first portion of this article explores key issues and principles in the perioperative management of surgical patients on warfarin therapy, and the second portion does the same for surgical patients on antiplatelet therapy.
ACCP RECOMMENDATIONS FOR PERIOPERATIVE ANTICOAGULANT MANAGEMENT
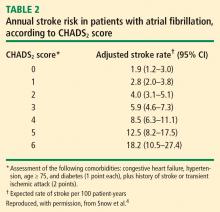
Patients with mechanical valves who are at high risk for perioperative thromboembolism include those with any mechanical mitral valve, an older valve, or a history of stroke or transient ischemic attack (TIA). Patients with atrial fibrillation who are at high risk include those with a recent stroke or TIA, rheumatic valvular heart disease, or a CHADS2 score of 5 or 6. (The CHADS2 scoring system assigns one point each for a history of congestive heart failure, hypertension, age greater than 75 years, or diabetes, and two points for history of stroke or TIA.) Patients with a history of VTE within the prior 3 months are also considered high risk.
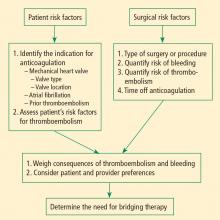
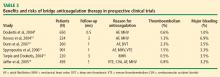
Bridging anticoagulation (bridge therapy)—ie, the temporary use of intravenous unfractionated heparin (IV UFH) or low-molecular-weight heparin (LMWH) prior to surgery—is central to the ACCP’s recommendations for perioperative management in patients on long-term anticoagulant therapy. Key ACCP recommendations1 for these patients, according to their risk for thromboembolism (Table 1), are as follows:
- High risk—bridging anticoagulation with therapeutic-dose subcutaneous LMWH or IV UFH
- Moderate risk—bridging anticoagulation with therapeutic-dose subcutaneous LMWH, therapeutic-dose IV UFH, or low-dose subcutaneous LMWH
- Low risk—bridging anticoagulation with low-dose subcutaneous LMWH or no bridging.
ASSESSING RISKS: DETERMINING WHETHER TO BRIDGE
Patient-specific risk factors
Patient risk factors include the indication for anticoagulation, as detailed above, as well as other individual risks for thromboembolism, as discussed in the article by Michota on preventing VTE on page S45 of this supplement.
If anticoagulation is indicated because the patient has a mechanical heart valve, the valve type and position must be considered because these factors affect thromboembolic risk, as reflected in Table 1. For instance, the risk of thromboembolism is greater when the valve is in the mitral position than in the aortic position, and is also greater with an older caged-ball valve than with a newer-generation bileaflet valve.3
Procedure-related risk factors
Surgical risks factors include the type of surgery and its associated risks of bleeding and thromboembolism, as well as the expected time that anticoagulation will be interrupted. Estimating thromboembolic risk is complicated, however, and reliable results are generally not achieved with simplistic calculations or formulas. Such calculations tend not to appropriately account for the hypercoagulable state induced by surgery itself, as the risk of VTE is estimated to be 100 times greater during the perioperative period than in the nonoperative setting, owing to increased levels of plasminogen activator inhibitor-1. Moreover, multiple studies have demonstrated increases in coagulation factors that suggest that a “rebound hypercoagulability” may occur shortly after discontinuation of oral anticoagulant therapy.5–8
Net benefit vs risk in trials of bridge therapy
In an analysis of data from observational studies, Kearon and Hirsh estimated the relative risk reduction for thromboembolism with bridge therapy to be 66% to 80%, depending on the indication for anticoagulation.8 Thus, if a patient’s risk of developing thromboembolism is 1.5%, bridge therapy reduces the risk to 0.5% or less.
Weigh relative consequences of an event with the patient
Determining whether and how to initiate bridge therapy ultimately depends on the consequences of an event. Recurrent VTE is fatal in 5% to 10% of cases,15 and arterial thromboembolism is fatal in 20% of cases and causes permanent disability in at least 50% of cases.16 While 9% to 13% of major bleeding events are fatal, bleeding rarely causes permanent disability.17 Thus, whereas a patient who bleeds can be resuscitated, a patient who develops a thromboembolism may be permanently disabled. These considerations should be shared with the patient, and patient preference should factor into the management strategy. Though the risk of bleeding with anticoagulation may be much higher than the risk of stroke without it, many patients will be more concerned about stroke risk.