Cardiac risk stratification for noncardiac surgery
ABSTRACT
The American College of Cardiology and American Heart Association updated their joint guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery in 2007. The guidelines recommend preoperative cardiac testing only when the results may influence patient management. They specify four high-risk conditions for which evaluation and preoperative treatment are needed: unstable coronary syndromes, decompensated heart failure, significant cardiac arrhythmias, and severe valvular disease. Patient-specific factors and the risk of the surgery itself are considerations in the need for an evaluation and the treatment strategy before noncardiac surgery. In most instances, coronary revascularization before noncardiac surgery has not been shown to reduce morbidity and mortality, except in patients with left main disease. The timing of surgery following percutaneous coronary intervention (PCI) depends on whether a stent was used, the type of stent, and the antiplatelet regimen.
KEY POINTS
- In addition to patient-specific factors, preoperative cardiac assessment should account for the risk of cardiac morbidity related to the procedure itself. Vascular surgery confers the highest risk, with reported rates of cardiac morbidity often greater than 5%.
- Continuation of chronic beta-blocker therapy is prudent during the perioperative period.
- Coronary revascularization prior to noncardiac surgery is generally indicated only in unstable patients and in patients with left main disease.
- Nonurgent noncardiac surgery should be delayed for at least 30 days after PCI using a bare-metal stent and for at least 365 days after PCI using a drug-eluting stent.
- Discontinuing antiplatelet therapy in patients with coronary stents may induce a hypercoagulable state within approximately 7 to 10 days.
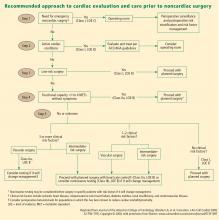
A FRAMEWORK FOR CARDIAC EVALUATION
The following are among the algorithm’s key recommendations:
- Patients requiring urgent noncardiac surgery should proceed to the operating room with perioperative surveillance (Class I, Level C).
- Patients with active cardiac conditions who are undergoing nonurgent surgery should be evaluated and treated per ACC/AHA guidelines before proceeding to the operating room is considered (Class I, Level B).
- Patients scheduled for a low-risk procedure can proceed to surgery without testing (Class I, Level B).
- Patients scheduled for intermediate-risk surgery or vascular surgery are to be assessed by functional capacity and clinical risk factors. Proceeding with planned surgery is appropriate in patients with good functional capacity (Class IIa, Level B). In patients with poor or unknown functional capacity undergoing vascular surgery who have three or more clinical risk factors, testing should be considered if the results would change management (Class IIa, Level B).
- Patients with one or more clinical risk factors undergoing intermediate-risk surgery and those with fewer than three clinical risk factors undergoing vascular surgery may proceed to planned surgery with control of heart rate to diminish the stress response perioperatively (Class IIa, Level B), or they may undergo noninvasive testing, but only if the results would change management (Class IIb, Level B).
- Patients undergoing intermediate-risk or vascular surgery who have poor or unknown functional capacity but no clinical risk factors may proceed to surgery without testing (Class I, Level B).
DISCUSSION
Question from the audience: The POISE study showed a 30% reduction in nonfatal MI with routine perioperative beta-blockade but an overall increase in mortality. Since most MIs occur immediately postoperatively and sepsis occurs a bit later, would you consider continuing beta-blocker therapy for a few days to prevent an MI but then stopping it before sepsis develops?
Dr. Fleisher: I’ve had discussions with sepsis experts about the link between beta-blocker therapy and sepsis and death in POISE, and the belief is that beta-blockers do not cause sepsis. I think that a septic patient on acute high-dose beta-blocker therapy can’t respond appropriately because of an inability to increase cardiac output. I believe we should titrate beta-blockers more closely. Information on preoperative dose titration in POISE is not available because of the way the trial was designed. Sepsis developed in only 53 of the 8,351 patients randomized in the study.
I would not start an acute beta-blocker protocol just to get a patient through surgery. I would start a perioperative hemodynamic protocol with the goal of maintaining the patient’s heart rate at lower than 80 beats per minute. Because I don’t believe that beta-blockers cause sepsis, if I initiated a beta-blocker preoperatively, I would not stop it at 2 days.
Question from the audience: Is there a time period during which a patient with a bare-metal stent could have back surgery or knee replacement surgery while not on aspirin?
Dr. Fleisher: The guidelines say that if a patient is on aspirin, it should be continued indefinitely. The issue is one of risk versus benefit. For back surgery, if bleeding is a concern, stopping aspirin for 6 or 7 days after the 30-day period following PCI is not unreasonable, but I would not stop it during the first 30 days following PCI.
Question from the audience: I don’t assess for vascular surgery but rather for the Whipple procedure [radical pancreatoduodenectomy], and I use the Revised Cardiac Risk Index to assess the number of risk factors. I believe the Whipple procedure is a high-risk operation, but it appears to be considered an intermediate-risk operation by the ACC/AHA guidelines. Is my approach to risk assessment appropriate?
Dr. Fleisher: If the rates of morbidity and mortality with the Whipple procedure are low at your institution, you might risk worsening your outcomes by applying someone else’s paradigms to your institution. There’s a big difference in risk between a surgeon who does a Whipple in 5 hours with 0.5 to 1.0 U of blood loss and a surgeon who does a 12-hour Whipple with 20 U of blood loss, necessitating a stay in the intensive care unit for multiple days. You need to consider the risk associated with your institution and specifically with the surgeon.
Question from the audience: Peripheral vascular disease is considered a coronary heart disease risk equivalent, so why is it not one of the criteria in the Revised Cardiac Risk Index?
Dr. Fleisher: The criteria are not hard and fast. The index was devised at one institution, Brigham and Women’s Hospital, in about 4,000 patients, and it has been used differently. It assigns 1 point to ischemic heart disease. It would not be inappropriate to assume that any atherosclerotic class of disease is equivalent to ischemic heart disease for risk purposes.
Question from the audience: You mentioned a 4-day window for withholding clopidogrel. Do you factor into the decision the duration of therapy? Some cardiologists go beyond the 1-year recommendation to continue clopidogrel after stenting because they believe there is still benefit.
Dr. Fleisher: The key is to confer with the cardiologist who implanted the stent, who knows the stenosis for which the stent was implanted. A problem we’ve had for years is that a practitioner will stop the antiplatelet agent without having spoken to the surgeon or the anesthesiologist. As an anesthesiologist, I need to know that someone has done a risk/benefit assessment of whether to continue antiplatelet agents in a given patient.
Question from the audience: The Revised Cardiac Risk Index of Lee et al3 includes the type of surgery in its total point system while the ACC/AHA guidelines do not. Can you explain the discrepancy?
Dr. Fleisher: We on the writing committee for the ACC/AHA 2007 perioperative guidelines made a decision to pull out the type of surgery and use the other five risk factors of Lee et al. It was a consensus of the committee because we believed that the complexity of the surgery itself is a separate consideration for risk. That’s why we included the medical risk factors and considered the surgical factors separately.