Novel antiplatelet strategies in acute coronary syndromes
ABSTRACT
Antiplatelet therapies for the treatment of acute coronary syndromes (ACS) act to interrupt various pathways of platelet activation. Clopidogrel, an established thienopyridine antiplatelet medication, inhibits adenosine diphosphate (ADP)–induced platelet aggregation to a modest degree and with wide variability in platelet response. Accumulating data suggest that a 600-mg loading dose of clopidogrel may help overcome the suboptimal response to the standard 300-mg dose seen in some patients. Prasugrel is a third-generation investigational thienopyridine that demonstrates more potent inhibition of platelet aggregation and more consistent platelet response compared with standard- and high-dose clopidogrel. A large clinical trial showed prasugrel to be superior to standard-dose clopidogrel in reducing ischemic events in patients with ACS scheduled for percutaneous coronary intervention, although prasugrel was associated with a significantly higher risk of major bleeding events. Other investigational antiplatelet agents also display more potent and consistent inhibition of platelet aggregation than is seen with clopidogrel. These include AZD6140, a reversible ADP receptor blocker; cangrelor, a rapidly acting intravenous ADP receptor blocker; and the thrombin receptor antagonist SCH 530348.
KEY POINTS
- There is substantial interpatient variability in the response to clopidogrel.
- In the large TRITON-TIMI 38 trial, the composite rate of death, myocardial infarction, or stroke was reduced by 19% and the rate of stent thrombosis was halved in patients receiving prasugrel compared with standard-dose clopidogrel.
- The risk of major bleeding with prasugrel is highest in patients aged 75 or older, those weighing less than 60 kg, and those with a history of stroke or transient ischemic attack.
- Thrombin receptor antagonists are being studied to see if their use can reduce ischemic events without increasing bleeding.
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
,Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
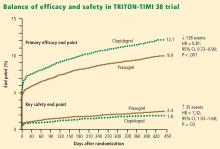
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17