Importance of platelets and platelet response in acute coronary syndromes
ABSTRACT
Platelet activation is one of the essential steps in the genesis and propagation of atherothrombosis. Accumulating clinical evidence suggests that an elevated platelet count, platelet activation, and platelet hyperreactivity (defined as residual platelet activity despite antiplatelet drug therapy) may be associated with adverse cardiovascular events in patients with acute coronary syndromes. Platelet function can be analyzed using various assays and measures of platelet activation. The best assays for measuring residual platelet activity in the setting of antiplatelet therapy are still being defined, as are their predictive values. Platelet aggregation remains the gold standard, but other testing methods offer advantages for specific applications, such as detecting overall platelet hyperreactivity in the presence of antiplatelet therapy or detecting inhibition of the adenosine diphosphate receptor P2Y12. Standard testing protocols for platelet aggregation are needed to achieve consistency among studies.
KEY POINTS
- Platelet function assays are inherently variable because they measure cell function rather than a single analyte.
- Screening tests, or global tests for platelet function, do not identify specific causes of platelet dysfunction but combine measurement of different aspects of platelet function.
- There appears to be a subgroup of patients with stable cardiovascular disease who have an increased risk of major cardiac events associated with platelet hyperreactivity.
- For predicting cardiac events, receiver operating characteristic (ROC) curve analysis should be used to objectively define cutoff values for platelet hyperreactivity as opposed to reliance on arbitrary cutoff values.
ROLE OF PLATELETS IN ACUTE CORONARY SYNDROMES: WHAT IS THE EVIDENCE?
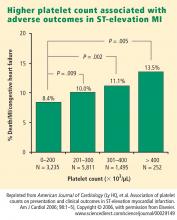
How predictive is an elevated platelet count?
However, another study conducted in a slightly different population—1,616 patients with non‑ST-segment-elevation MI/unstable angina—found no correlation between platelet count (by quintiles) and death at 60 months.3 The lowest mortality was observed in patients with a platelet count in the second-lowest quintile, although the highest mortality was indeed observed in the quintile of patients with the lowest platelet counts.3
The differing results in the above two studies suggest that additional platelet factors, beyond platelet count, contribute to the risk of adverse outcomes following ACS.
Platelet hyperreactivity and outcomes in ACS
Platelet hyperreactivity—ie, residual platelet activity despite antiplatelet therapy—appears to be involved in the spectrum of ACS. A recent study evaluated the association between hyperreactivity of platelets to ADP and outcomes in 600 patients with stable cardiovascular disease who were on aspirin therapy.4 Hyperreactivity was defined as a collagen/ADP closure time of less than 90 seconds on the PFA-100 system (short collagen/ADP closure time). On receiver operating characteristic (ROC) curve analysis, a short collagen/ADP closure time served as a significant predictor of recurrent events (relative risk [RR] = 3.65; 95% CI, 1.76–7.57) and death (RR = 6.56; 95% CI, 1.93–22.35) compared with a closure time of 90 seconds or greater. The authors concluded that there appears to be a subgroup of patients with stable cardiovascular disease who have an increased risk of major adverse events associated with platelet hyperreactivity.4
An earlier study by Harrison et al assessed platelet function using the PFA-100 in 78 patients presenting with acute chest pain classified as MI, unstable angina, or nonspecific chest pain.5 Using the PFA-100, they found shorter collagen/ADP closure times and higher levels of von Willebrand factor in subjects with MI compared with those who had unstable angina or nonspecific chest pain.5 Fuchs et al reported a similar association between von Willebrand factor and outcomes in 208 patients with ACS,6 raising the possibility that von Willebrand factor, through its association with increased platelet adhesion and activation, may be a major contributor to risk in ACS.
Similarly, an association between platelet hyperreactivity and cardiovascular events has been suggested in patients with type 2 diabetes. In a 2007 study of 173 patients with type 2 diabetes and coronary artery disease receiving dual antiplatelet therapy (aspirin plus clopidogrel), the 2-year risk of major cardiovascular events was significantly higher in those in the highest quartile of platelet aggregation compared with those in the lower three quartiles (hazard ratio = 3.35; 95% CI, 1.68–6.66).7 In a separate study, Serebruany et al measured platelet activity by five different testing methods in 822 patients with coronary artery disease and found significantly higher platelet hyperreactivity by all methods in those patients who had diabetes (n = 257) than in those who did not (n = 565).8
Marcucci et al recently examined the relationship between clinical characteristics and residual platelet activity in 386 patients with ACS on dual antiplatelet therapy (aspirin plus clopidogrel).9 The presence of residual platelet activity (determined by platelet aggregation in response to the agonists arachidonic acid and ADP, as well as by the PFA-100) was associated with significantly higher inflammatory status, as determined by leukocyte count and erythrocyte sedimentation rate. The same association was observed among a subset of patients in this study undergoing percutaneous coronary intervention (PCI) who were receiving dual antiplatelet therapy; additionally, residual platelet activity was associated with a significantly higher incidence of diabetes and a significantly lower ejection fraction in this subset.9
Platelet hyperreactivity while on dual antiplatelet therapy (aspirin plus clopidogrel) was also found to be predictive of clinical outcome in a study of 195 patients with non-ST-elevation MI undergoing PCI.10 Hyporesponse to antiplatelet therapy, as measured by a high VASP platelet reactivity index (PRI), predicted an increased risk of recurrent ischemic events within 30 days of PCI. Using ROC curve analysis, the investigators found that a VASP PRI cutoff value of 53% (ie, a high PRI [> 53%] indicates residual platelet activity despite clopidogrel) had a sensitivity of 93%, a specificity of 50%, a positive predictive value of 12%, and a negative predictive value of 99% for ischemic events.10 Similarly, among 144 patients undergoing PCI assessed for decreased platelet reactivity to a loading dose of clopidogrel, Bonello et al also found that a VASP PRI greater than 50% was optimal for predicting major adverse cardiovascular events: all 21 events in the study occurred among patients whose VASP PRI was in the highest four quintiles.11
CONCLUSIONS AND GENERAL ASSESSMENT OF PLATELET FUNCTION TESTS
Platelets clearly are involved in the pathogenesis of atherothrombosis. Accumulating evidence suggests that both an elevated platelet count and platelet hyperreactivity (residual platelet activity despite dual antiplatelet therapy) may be associated with adverse cardiovascular events in patients with ACS.
Platelet function can be measured using several different assays and measures of platelet activation. The best assays for measuring residual platelet activity in the setting of antiplatelet therapy are still being defined, as are their predictive values. Platelet aggregation remains the gold standard. The PFA-100 may detect overall platelet hyperreactivity despite the use of antiplatelet therapy, and is attracting increasing use for this purpose. VASP phosphorylation may be a good assay for detecting P2Y12 inhibition but is limited to thienopyridines in terms of detecting platelet hyperreactivity. For predicting adverse cardiac events, ROC curve analysis should be used to objectively define cutoff values for platelet hyperreactivity as opposed to reliance on arbitrarily defined cutoff values.
Moving forward, standard testing protocols for platelet aggregation clearly are needed to achieve consistency among studies.