Importance of platelets and platelet response in acute coronary syndromes
ABSTRACT
Platelet activation is one of the essential steps in the genesis and propagation of atherothrombosis. Accumulating clinical evidence suggests that an elevated platelet count, platelet activation, and platelet hyperreactivity (defined as residual platelet activity despite antiplatelet drug therapy) may be associated with adverse cardiovascular events in patients with acute coronary syndromes. Platelet function can be analyzed using various assays and measures of platelet activation. The best assays for measuring residual platelet activity in the setting of antiplatelet therapy are still being defined, as are their predictive values. Platelet aggregation remains the gold standard, but other testing methods offer advantages for specific applications, such as detecting overall platelet hyperreactivity in the presence of antiplatelet therapy or detecting inhibition of the adenosine diphosphate receptor P2Y12. Standard testing protocols for platelet aggregation are needed to achieve consistency among studies.
KEY POINTS
- Platelet function assays are inherently variable because they measure cell function rather than a single analyte.
- Screening tests, or global tests for platelet function, do not identify specific causes of platelet dysfunction but combine measurement of different aspects of platelet function.
- There appears to be a subgroup of patients with stable cardiovascular disease who have an increased risk of major cardiac events associated with platelet hyperreactivity.
- For predicting cardiac events, receiver operating characteristic (ROC) curve analysis should be used to objectively define cutoff values for platelet hyperreactivity as opposed to reliance on arbitrary cutoff values.
PLATELET FUNCTION TESTS
Platelet function assays are inherently variable because they measure cell function rather than a single analyte. Several new platelet testing devices have come to market with the goal of ease of use; many can now be used at the bedside to measure platelet function.
Platelet count
In my view, the platelet count remains one of the best tests for assessing bleeding risk, as a low platelet count is one of the most common causes of bleeding. However, the platelet count is not a functional assay because it does not evaluate other platelet functions.
Screening tests
Screening tests, or global tests for platelet function, do not identify specific causes of platelet dysfunction but combine measurement of many different aspects of platelet function, such as adhesion, aggregation and granule release.
Bleeding time. The bleeding time is an archaic test because of the poor correlation between bleeding time and bleeding disorders or thrombotic disorders. Its utility in measuring platelet function is therefore highly limited.
PFA-100. The PFA-100 Platelet Function Analyzer system (PFA-100) is one example of a global platelet function assay that measures multiple platelet functions, including platelet adhesion and aggregation. The instrument, which is about the size of a bread box, uses a citrate-anticoagulated whole blood specimen to measure platelet reaction in a high-shear environment. Blood travels at high shear rates through membranes coated with either collagen and ADP or collagen and epinephrine (epinephrine receptors exist on platelet surfaces). Platelets adhere to the membranes and then activate, aggregate, and occlude a small aperture in the center of each membrane, yielding a measurable closure time.
Since the PFA-100 was developed before the availability of the thienopyridine antiplatelet drugs, its utility lies not in monitoring the effects of those agents but in its ability to detect aspirin-induced platelet dysfunction or intrinsic platelet function disorders. An abnormal epinephrine cartridge closure time in the presence of a normal ADP cartridge closure time indicates aspirin-induced platelet dysfunction. An abnormal closure time on both measures is indicative of von Willebrand disease or a platelet defect such as Glanzmann thrombasthenia or Bernard-Soulier syndrome.
Specific functional tests
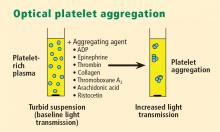
Whole blood platelet aggregation is typically a high-complexity laboratory test. Recently, self-contained assay platforms that can measure whole blood aggregation have been developed. These are applicable for smaller hospitals and near-patient settings. One such rapid platelet function analyzer, known commercially as VerifyNow, offers point-of-care assessment of platelet function. The instrument, which is the size of a telephone answering machine, operates by a principle similar to that of optical platelet aggregation: platelet function is measured by the rate and extent of change in light transmittance in response to the introduction of agonists specific to various antiplatelet medications. Low light transmittance indicates a blood sample with inhibited platelet function; high light transmittance indicates normal platelet function.
Measurement of VASP phosphorylation. Vasodilator-stimulated phosphoprotein (VASP) is an intracellular platelet protein that is nonphosphorylated in basal state. The phosphorylation of VASP depends on the level of activation of the P2Y12 receptor, a target of thienopyridine drugs. Thus, measuring VASP phosphorylation by flow cytometry using citrated whole blood can be a highly specific indicator of the action and efficacy of clopidogrel and other thienopyridine drugs.
A flow cytometry assay that measures VASP phosphorylation requires a whole blood sample that is incubated with ADP to measure what is called the platelet reactivity index. Adding ADP to whole blood stimulates adenylate cyclase, lowering cAMP and shutting off protein kinase, which results in low levels of VASP phosphorylation. Thus, if VASP is phosphorylated, the platelets are inhibited; if VASP is not phosphorylated, the platelets are activated. A satisfactory therapeutic response to clopidogrel or another thienopyridine drug produces a low platelet reactivity index, reflecting platelet inhibition.
ROLE OF PLATELETS IN ATHEROSCLEROSIS
Platelets serve major functions in three key aspects of atherosclerosis: atherogenesis, inflammation, and atherothrombosis.
Atherogenesis
Platelets play a pivotal role in atherogenesis.1 They release matrix metalloproteinases that are involved in degrading the matrix in atherosclerotic plaques. Moreover, they contain and release chemokines and growth factors, including:
- RANTES, a chemokine that stimulates monocytes and T cells to increase the production of monocyte inflammatory mediators
- Platelet-derived growth factor, which stimulates the migration and proliferation of smooth muscle cells
- Transforming growth factor–β, which also stimulates proliferation of smooth muscle cells.
Inflammation
Activated platelets release inflammatory mediators and thereby change the adhesive and chemotactic properties of endothelial cells. Likewise, mediators derived from inflammatory cells (neutrophils) can affect platelet function.
Platelet-derived mediators include the following:
- Pro‑interleukin (IL)-β, which triggers the synthesis of E-selectin that enables endothelial cells to interact with leukocytes
- Thromboxane A2, which increases neutrophil adhesion to facilitate platelet aggregation
- Platelet-derived growth factor and platelet factor 4, which increase neutrophil chemotaxis (the ability of neutrophils to infiltrate atherosclerotic plaque)
- CD40 ligand, a protein expressed on platelets that induces inflammatory responses in the endothelium
- P-selectin, a cell adhesion molecule expressed on activated platelets that enhances the adhesion of monocytes on activated endothelial cells.
Among the neutrophil-derived mediators, some—such as superoxide and leukotrienes—enhance platelet activation, whereas elastases inhibit platelet activation.
Overall, once inflammation begins in an atherosclerotic plaque, much reciprocal platelet activation can occur, so that the inflammatory process can become a feed-forward loop to eventually promote atherothrombosis.
Atherothrombosis
In the last stage of the atherosclerotic process, platelet enzymes that degrade the matrix may make plaques vulnerable to rupture by creating fissures in the fibrous plaque cap. This exposes the lipid-rich core, which contains a significant amount of thromboplastin. Exposure to the extracellular matrix can lead to further platelet adhesion, activation, and aggregation. The development of a platelet thrombus is usually one of the ultimate steps in atherothrombosis leading to ACS, including MI.