Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
ABSTRACT
Patients with soft-tissue sarcomas generally present with a mass that is increasing in size; the presence or absence of pain is not typically predictive of malignancy. While magnetic resonance imaging (MRI) can identify a few soft-tissue lesion types as benign, diagnosis of most lesions requires a careful biopsy, preferably performed by or in consultation with the surgeon who would do an eventual resection. If biopsy confirms a diagnosis of sarcoma, MRI-guided surgical resection with a wide margin is the mainstay of treatment. Neoadjuvant radiation therapy and chemotherapy have not been of proven benefit in well-controlled studies but are frequently used as adjuncts. Resections with wide margins are generally associated with a low (< 10%) risk of recurrence.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
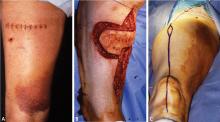
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.