A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds
ABSTRACT
Drug-eluting stents (DES) are increasingly being used as a less invasive alternative to coronary artery bypass grafting. Early generation DES had durable polymers that provided acceptable efficacy outcomes but had high rates of stent thrombosis leading to myocardial infarction and death. Second-generation DES have improved outcomes by reducing stent thrombosis and recurrent stenosis. Newer DES with biodegradable polymers have similar efficacy as second-generation DES, but have higher rates of stent thrombosis. This review compares outcomes of bioresorbable scaffolds and looks at stent technology developments that may improve outcomes.
KEY POINTS
- Complications with first-generation durable polymer DES—stent thrombosis and restenosis with target lesion revascularization—led to the development of bioresorbable stents.
- Bioresorbable and durable polymer metallic DES have similar rates of efficacy and of stent thrombosis.
- Bioresorbable DES should be placed in appropriate patient populations and lesion subsets, and limited to arteries larger than 2.25 mm.
ABSORB II STUDY RESULTS RAISE QUESTIONS
Another concern was uncovered in July 2016 when results were published from the ABSORB II trial on vasomotor reactivity at 3 years.13 This clinical trial randomized 501 patients in a 2:1 ratio to the Absorb BVS or the Xience DES at 46 sites outside the United States. Assessment for changes in mean lumen diameter between pre- and post-nitrate administration showed no differences between the groups; thus, the Absorb BVS did not achieve a level of superior vasomotor reactivity. There was vasomotor reactivity probably because the surrogate marker was angiographic follow-up and not intravascular ultrasound or tomography.
Further, the coprimary end point of angiographic late luminal loss at 3 years did not meet its noninferiority standard. The Absorb BVS was expected to have lower rates of late lumen loss because the struts are gone and there is less new intimal formation; however, at 3 years, that was not the case.
The rate of acute stent thrombosis also was alarming: 8 cases for Absorb BVS versus none for Xience. This caused alarm, raising the question of why it was happening in these patients 2 to 3 years after implantation.
Animal studies investigating the association of thicker struts and increased thrombogenicity have reported that the 157-µm BVS had much more platelet buildup and thrombogenicity than a 120-µm biomatrix stent. The 74-µm Synergy stent had even lower rates of thrombosis. The reason for increased thrombogenicity with thicker struts requires further study.
Also, an analysis of the secondary cardiac end points at 3 years in ABSORB II found no clinical patient-oriented differences between the Absorb BVS and the Xience stent (20.8% vs 24.0%, respectively; P = .44). However, rates of device-oriented clinical end points were significantly higher for Absorb BVS (10.4% vs 4.9%; P = .043).13
Clearly, the results for Absorb BVS in this study were not positive. One explanation is suboptimal implantation techniques that did not appose the polymer to the wall. A few years ago, focus shifted to an optimal technique for scaffold deployment, which included predilation, appropriate sizing of the scaffold to the size of the vessel, and postdilation with the intention of embedding the polymer in the vessel wall. Multiple studies have reported fewer incidents of stent thrombosis with the implementation of this protocol.14
Further studies have continued to report increased rates of late scaffold thrombosis in follow-ups of 30 days to 3 years. This resulted in an advisory letter from the FDA focused on appropriate clinical use of the device and withdrawal of ABSORB from commercial use in Europe and Australia.
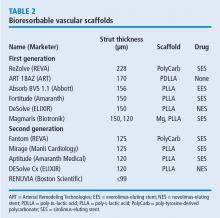
BIORESORBABLE SCAFFOLDS PIPELINE
This is questionable because one has to believe in the vulnerable plaque theory, which assumes potential eruption of plaques. The Absorb can actually seal a thin cap atheroma and necrotic core over time. It seems that this technology can cause some late lumen enlargement and seal an existing plaque, which may have implications for the future.
SUMMARY
This is the current state of the Absorb BVS:
- More than 150,000 implanted globally
- Received FDA approval in July 2016
- Should not be used in small vessels (ie, lumen diameter < 2.25 mm)
- Thrombosis rates 2 to 3 years after implantation are of concern
- Focusing on appropriate surgical implantation technique can improve outcomes.
Overall, use of bioresorbable stent technology is intriguing. While there is ongoing patient preference for bioresorbable technology, clinical trial results raise the question of whether bioresorbable scaffolds are inferior to best-in-class DES. Improving the scaffold technology and the implantation techniques may equate the short-term outcome of the bioresorbable scaffolds with metallic stents with the hope that over time (when the scaffold is gone), the advantage will be with the bioresorbable scaffolds. Meanwhile, the technology is still seeking its best clinical utility, and a matching performance to the best-in-class DES.
Time will tell whether 5 to 10 years after implantation, BRS technology will outperform durable metallic stents.