A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds
ABSTRACT
Drug-eluting stents (DES) are increasingly being used as a less invasive alternative to coronary artery bypass grafting. Early generation DES had durable polymers that provided acceptable efficacy outcomes but had high rates of stent thrombosis leading to myocardial infarction and death. Second-generation DES have improved outcomes by reducing stent thrombosis and recurrent stenosis. Newer DES with biodegradable polymers have similar efficacy as second-generation DES, but have higher rates of stent thrombosis. This review compares outcomes of bioresorbable scaffolds and looks at stent technology developments that may improve outcomes.
KEY POINTS
- Complications with first-generation durable polymer DES—stent thrombosis and restenosis with target lesion revascularization—led to the development of bioresorbable stents.
- Bioresorbable and durable polymer metallic DES have similar rates of efficacy and of stent thrombosis.
- Bioresorbable DES should be placed in appropriate patient populations and lesion subsets, and limited to arteries larger than 2.25 mm.
THE RATIONALE FOR BIORESORBABLE STENTS
Another approach was to use biodegradable scaffolds that will be eliminating from the vessel wall once it “completes the job.” The main bioresorbable materials used were polylactic acid or biodegradable metal-like magnesium. These materials pose a technological challenge. While the biodegradable scaffolds are completely eliminated overtime, they still need to equate the performance of best-in-class drug-eluting stent with respect to efficacy and safety. After the Absorb everolimus-eluting BVS system (Absorb BVS) was launched in Europe, initial studies showed scaffold-related thrombosis rates as high as 3.4%.5–7 That compares with 0.4% for second-generation DES—a troubling result for a new technology.
Rates of restenosis and stent thrombosis are similar for bioresorbable stents and standard durable polymer stents. But what are the potential added benefits of bioresorbable stents? And will they improve patient outcomes?
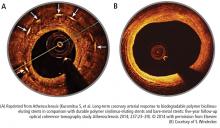
Bioresorbable stents certainly appeal to patients who do not want a permanent, rigid, metallic implant. Also appealing are the proposed benefits of restoration of vasomotion, late luminal enlargement, preservation of CABG targets, and relief of angina. Whether bioresorbable stents improve these outcomes has not been established. Currently, there is no long-term evidence of reduced rates of adverse events, although in 1 study, optical coherence tomography images recorded 10 years after implantation of the first bioresorbable stents showed a pristine vessel with no signs of the struts.8
Several facts are known about the Absorb BVS:
- Preclinical evidence shows complete resorption and return of vascular function, but this takes 3 to 4 years.
- Imaging data at 5 years from the Absorb cohort B trial show complete resorption of struts, lumen preservation, return of function, and plaque regression.9
- In ABSORB III, the pivotal US trial, the stent was within the primary end point showing noninferiority in safety and effectiveness compared with Xience in the first year.10
- Absorb clinical trials in Japan and China confirmed ABSORB III results.
- Meta-analysis (> 3,300 patients) confirmed safety and effectiveness of Absorb.11
- Real-world Absorb clinical evidence continues to show improving outcomes with optimized implant techniques.
- Absorb stent was approved by the US Food and Drug Administration (FDA) in July 2016; more than 150,000 have been implanted worldwide.
The increased rates of target-lesion revascularization and stent thrombosis were likely attributable to inserting the stents into small-diameter vessels that are probably too small for the Absorb BVS. When small vessels (< 2.25 mm) are eliminated from the analysis, the rates were as follows.
Results for vessels > 2.25 mm:
- Target-lesion revascularization: 6.7 % vs 5.5%
- Stent thrombosis: 0.9% vs 0.6%.
- Results for small vessels (< 2.25 mm):
- Target-lesion revascularization: 12.9% vs 8.3%
- Stent thrombosis: 4.6% vs 1.5%.
The lesson is that the Absorb BVS should not be placed in arteries smaller than 2.25 mm in diameter.