A minimally invasive treatment for early GI cancers
ABSTRACT
Endoscopic submucosal dissection (ESD) allows curative resection of early malignant gastrointestinal (GI) lesions, potentially avoiding open surgery. Unfortunately, awareness of this technique is low, and many patients undergo surgery without consideration of ESD. This article reviews the indications for ESD and its advantages and limitations, and guides internists in their approach to patients with early GI cancer.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
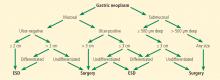
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
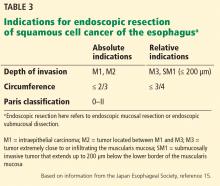
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5