PCSK9 inhibition: A promise fulfilled?
ABSTRACTThe association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced cardiovascular disease (CVD) events—and the need for add-ons to statin therapy to achieve treatment goals—has led to the rapid development and US Food and Drug Administration (FDA) approval of monoclonal antibody therapies to inhibit PCSK9. Now that PCSK9 inhibitors are approved by the FDA for use in certain patients, data from ongoing long-term clinical trials addressing tolerability, safety, and proof of additional reduction in CVD events are eagerly awaited
KEY POINTS
- Potential candidates for PCSK9 inhibitor therapy are patients with familial hypercholesterolemia with a lifetime burden of elevated low-density-lipoprotein cholesterol (LDL-C) and thus a low likelihood of optimal control on current therapies; patients with complete or partial statin intolerance, with high-intensity statin dosing limited by adverse effects; and patients at high CVD risk with LDL-C goals not achieved with current therapies.
- Subcutaneously administered monoclonal antibodies targeting PCSK9 are currently the only PCSK9 inhibitors with FDA approval.
- PCSK9 inhibitors under study include agents with more durable effect and that require less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
,A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
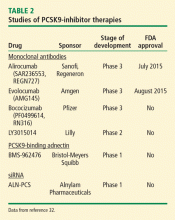
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
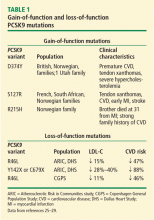
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32