Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
ABSTRACTStroke prevention in patients with nonvalvular atrial fibrillation relies on an assessment of the individual risks for stroke and bleeding. Patients at high risk for stroke are candidates for anticoagulant therapy. Anticoagulants, however, have substantial bleeding risks that must be weighed in the therapeutic decision. Warfarin has been the traditional choice, but the recently introduced novel oral anticoagulants offer similar efficacy with less bleeding risk. Additionally, they do not require monitoring and have fewer drug interactions and dietary restrictions than warfarin. Several devices, which isolate the left atrial appendage, have become available as treatment options for patients with elevated risks of both thromboembolism and bleeding complications.
KEY POINTS
- Specific risk factor management is as important as anticoagulation when addressing stroke risk.
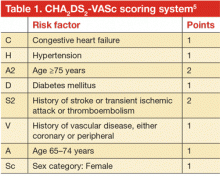
- The CHADS2 score has been superseded by the CHA2DS2-VASc score, which is more accurate for lower-risk categories.
- Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
- Most atrial thrombi in patients with nonvalvular atrial fibrillation form in the left atrial appendage (LAA); nonpharmacologic interventions have been developed to block the LAA and reduce the risk of stroke.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12