Atrial Fibrillation and Bleeding in Patients With Chronic Lymphocytic Leukemia Treated with Ibrutinib in the Veterans Health Administration
Background: Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in adults. The introduction of novel oral agents, starting with ibrutinib in 2013, has revolutionized the therapeutic landscape; however, clinical trials have suggested an association between ibrutinib and the risk of bleeding-related adverse events and atrial fibrillation (Afib) in patients with CLL.
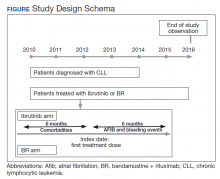
Methods: Patients diagnosed and treated for CLL at the Veterans Health Administration (VHA) from 2010 to 2014 were followed until December 31, 2016, death, or lack of utilization of hematology/oncology services for ≥ 18 months; or until incidence of another cancer. Treatments dispensed, evidence of VHA system use, bleeding events, and Afib were determined from the administrative records, laboratory records, pharmacy dispensation records, and clinical notes in the electronic healthcare record.
Results: From 2010 to 2014, 2,796 patients were diagnosed and received care for CLL within the VHA, of whom 172 patients received ibrutinib and 291 received bendamustine + rituximab (BR). The use of anticoagulants following induction therapy did not differ between BR and ibrutinib patients (9% vs 8%, respectively), nor did the use of antiplatelets agents (6% vs 2%, respectively). Of the 291 patients that received BR, 12 (4%) developed a bleeding event compared with 20 (12%) who received ibrutinib. Additionally, 13 (8%) ibrutinib patients developed Afib compared with 9 (3%) BR patients.
Conclusions: Real-world evidence from a nationwide cohort of patients with CLL suggests that while ibrutinib is associated with increased bleeding-related adverse events and Afib, the risk is comparable to those reported in previous clinical trials. These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at an increased risk for bleeding events and Afib.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.