News
sNDA gets priority review for CLL/SLL
- Author:
- HT Staff
Publish date: October 18, 2018
News
STRO-001 receives orphan designation for MM
- Author:
- HT Staff
Publish date: October 14, 2018
News
FDA approves test to determine blood compatibility
- Author:
- HT Staff
Publish date: October 13, 2018
News
Inhibitor receives orphan designation for PTCL
- Author:
- HT Staff
Publish date: October 11, 2018
News
Phase 1 NHL, ALL trials placed on clinical hold
- Author:
- HT Staff
Publish date: October 10, 2018
News

Selinexor receives priority review for penta-refractory MM
- Author:
- HT Staff
Publish date: October 9, 2018
News
Researchers say rethink ‘arbitrary categorization’ of VTE risk
- Author:
- HT Staff
Publish date: October 8, 2018
News

Duvelisib bests ofatumumab as monotherapy for treatment of CLL/SLL
- Author:
- HT Staff
Publish date: October 5, 2018
News
Emicizumab now also approved for hemophilia A without inhibitors
- Author:
- HT Staff
Publish date: October 5, 2018
News
Researchers develop genetics-based prognostic tool for MDS
- Author:
- HT Staff
Publish date: October 3, 2018
News
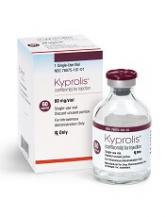
Carfilzomib receives approval for once-weekly dosing
- Author:
- HT Staff
Publish date: October 3, 2018
News
Single leukemic cell can contaminate CAR T-cell product
- Author:
- HT Staff
Publish date: October 2, 2018
News

Two immunologists receive Nobel Prize in medicine
- Author:
- HT Staff
Publish date: October 1, 2018
News
First NGS assay approved for MRD detection in ALL or MM
- Author:
- HT Staff
Publish date: September 29, 2018
News
Auto-FMT restores gut microbiota after HSCT
- Author:
- HT Staff
Publish date: September 28, 2018