2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Interstitial cystitis/bladder pain syndrome is difficult to diagnose. Here, updated guidelines on recognition, with tools to help the cause, and data on promising secondary treatments.
In this Article
- AUA diagnosis guidelines
- Treatment algorithm
- A new FDA-approved oral treatment option
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
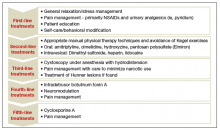
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.