Overcoming barriers to clinical trial enrollment in patients with bone and soft tissue sarcoma: a paradigm for an increasingly heterogeneous cancer population
Abstract
Background: Clinical trials remain a cornerstone in the development of new cancer therapies. Patients diagnosed with bone and soft tissue sarcoma typically have comparatively low rates of trial participation, which may contribute to lack of progress. This cross-sectional survey study was designed to characterize patient attitudes, knowledge, perceived ability (ie, self-efficacy), receptivity, and willingness to participate in trials. As well, we explored perceptions related to tumor molecular profiling (TMP).
Methods: Patients with sarcoma who were evaluated at Northwestern Medicine (NM) between 2012 and 2017 were identified through the Enterprise Data Warehouse (NMEDW). A link to an online self-administered survey was emailed to patients. Data were analyzed using Spearman correlations and the Mann-Whitney test.
Results: Surveys were e-mailed to 750 patients, of which 20 emails bounced back and 309 were opened; 283 patients completed at least a portion of the survey and this population was used in this analysis (37.7% of total and 91.6% of opened). Of the responding patients, median age was 56 years, 59.4% were female, and 26.8% reported metastatic disease. Greater knowledge of trials correlated with increased positive attitudes toward trial participation (r=0.5; P<0.001) and positive attitudes correlated with greater trial perceived ability (r=0.4; P<0.02). Patients with metastatic disease had more positive attitudes compared with patients with non-metastatic disease (P=0.03). Trial enrollment was associated with greater knowledge (P=0.002) and positive attitudes (P=0.001). Among patients who reported knowledge of TMP (n=46), 65% credit TMP with a >50% chance of isolating a targetable result. Of patients who had TMP performed (n=18), important considerations included wanting to live as long as possible and helping future cancer patients, but not wanting to be part of research.
Conclusions: Increasing awareness, knowledge, and attitudes towards trials among patients with sarcoma may lead to increased trial enrollment and greater progress in cancer treatment. Patient-focused interventions and research-site optimization are critical to maximizing accrual rates and biomarker-driven therapeutics. Lessons from a sarcoma patient population can be used to optimize trial enrollment in an era of increasingly heterogeneous cancer populations.
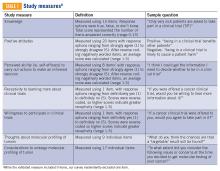
Clinical trial perceptions included questions assessing (1) patient knowledge about trials; (2) patient attitudes toward trials; (3) perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about trial participation; (4) receptivity to learning more about trials; and (5) willingness to participate in trials. These outcome measures had been previously developed and pilot tested for reliability and validity (TABLE 1).6
Thoughts about molecular profiling of tumors were assessed using nine items (TABLE 1). Of these, items assessing potential benefit or harm of molecular profiling were assessed using a 7-step Likert scale. Items assessing maximal benefit or harm of therapy, importance of quality vs length of life, and concern about the cost of molecular testing were assessed using a 5-step Likert scale. The study team developed and piloted these questions because there is no validated survey assessing these domains.
Considerations to undergo molecular profiling were assessed using 17 items. Items were in response to the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” Responses were assessed using a 5-point Likert scale.
Data Collection
Patients 18 years and older evaluated at NM between November 20, 2012, and November 20, 2017, with a diagnosis of sarcoma were identified by query of the NMEDW by ICD-10 codes (C40, C41.9, C44.99, C45-49, C55, C71.9, D48, D49.9, and M12.20) or equivalent ICD-9 codes. Patients were subsequently excluded if they did not have a diagnosis of bone or soft tissue sarcoma, no e-mail address listed, had died, or had not been evaluated at an NM clinic in the previous 5 years. Patients with a diagnosis of gastrointestinal stromal tumor and Kaposi’s sarcoma were also excluded.
A personalized contact e-mail was sent to patients containing an explanation of the survey and an internet link to the electronic survey through REDCap from January 2018 to March 2018. If patients did not respond to the survey, two follow-up reminder e-mails were sent 2 and 4 days following the initial survey. The link was protected so that each patient could complete the survey only once. Responses were collected through the REDCap platform. Patients read and signed an electronic consent form prior to completing the survey.
Upon completion of the survey, patients were offered a $50 VISA gift card as compensation, with an option to donate their compensation to the Robert H. Lurie Comprehensive Cancer Center Sarcoma Research Fund.
Over the described survey period, open clinical trials for patients with bone and soft tissue sarcoma available at NM were evaluated. The number of patients screened and accrued to each trial were recorded.