Stable COPD: Initiating and Optimizing Therapy
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects.1-4 It is a major cause of morbidity and mortality, affecting 5% of the population in the United States and ranking as the third leading cause of death in 2008.5,6 The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this 3-part review, we discuss the management of stable COPD in the context of 3 common clinical scenarios: initiating and optimizing therapy, managing acute exacerbations, and managing advanced disease.
Case Presentation
A 65-year-old man with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect: forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC), 0.45; FEV1, 2 L (65% of predicted); and diffusing capacity of the lung for carbon monoxide, 15 mL/min/mm Hg (65% of predicted). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the past year, and this was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
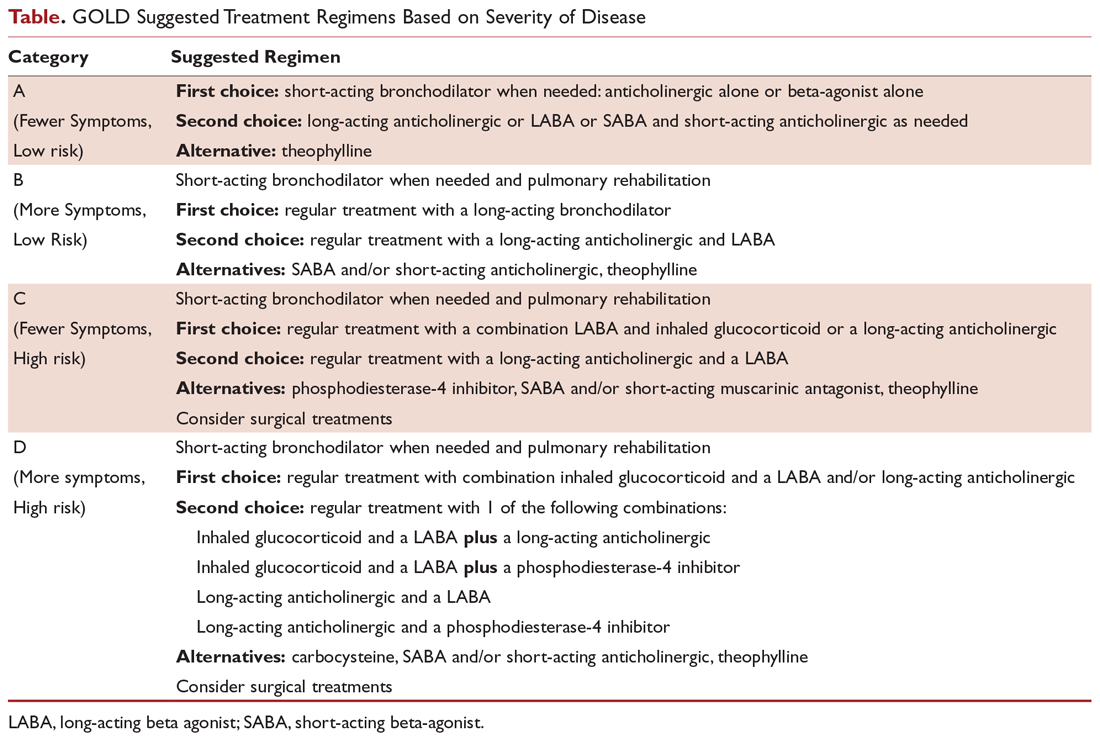
COPD management is guided by disease severity that is measured using a multimodal staging system developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The initial classification adopted by the GOLD 2011 report encompassed 4 categories based on symptoms, number of exacerbations, and degree of airflow limitation on PFT. However, in 2017 the GOLD ABCD classification was modified to consider only symptoms and risk of exacerbation in classifying patients, regardless of performance on spirometry and FEV1 (Figure 1).7,8 This approach was intended to make therapy more individualized based on the patient clinical profile. The Table provides a summary of the recommended treatments according to classification based on the GOLD 2017 report.
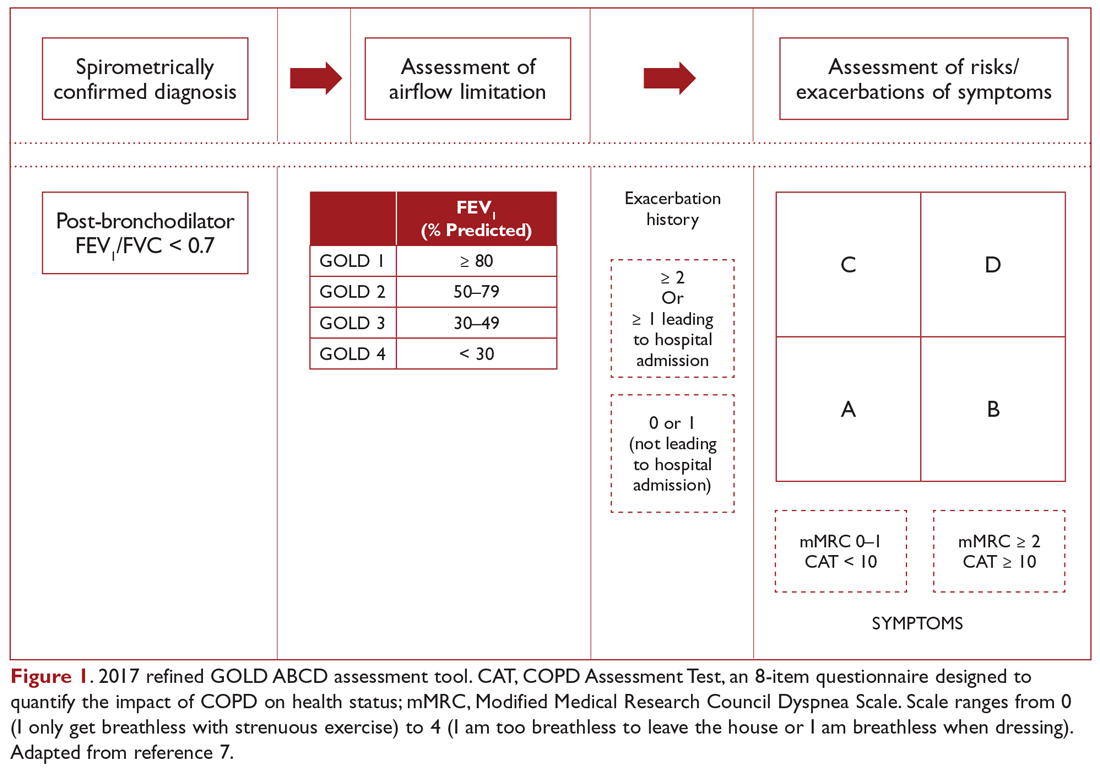
The patient in our clinical scenario can be classified as GOLD category B.
What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta-agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta- agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
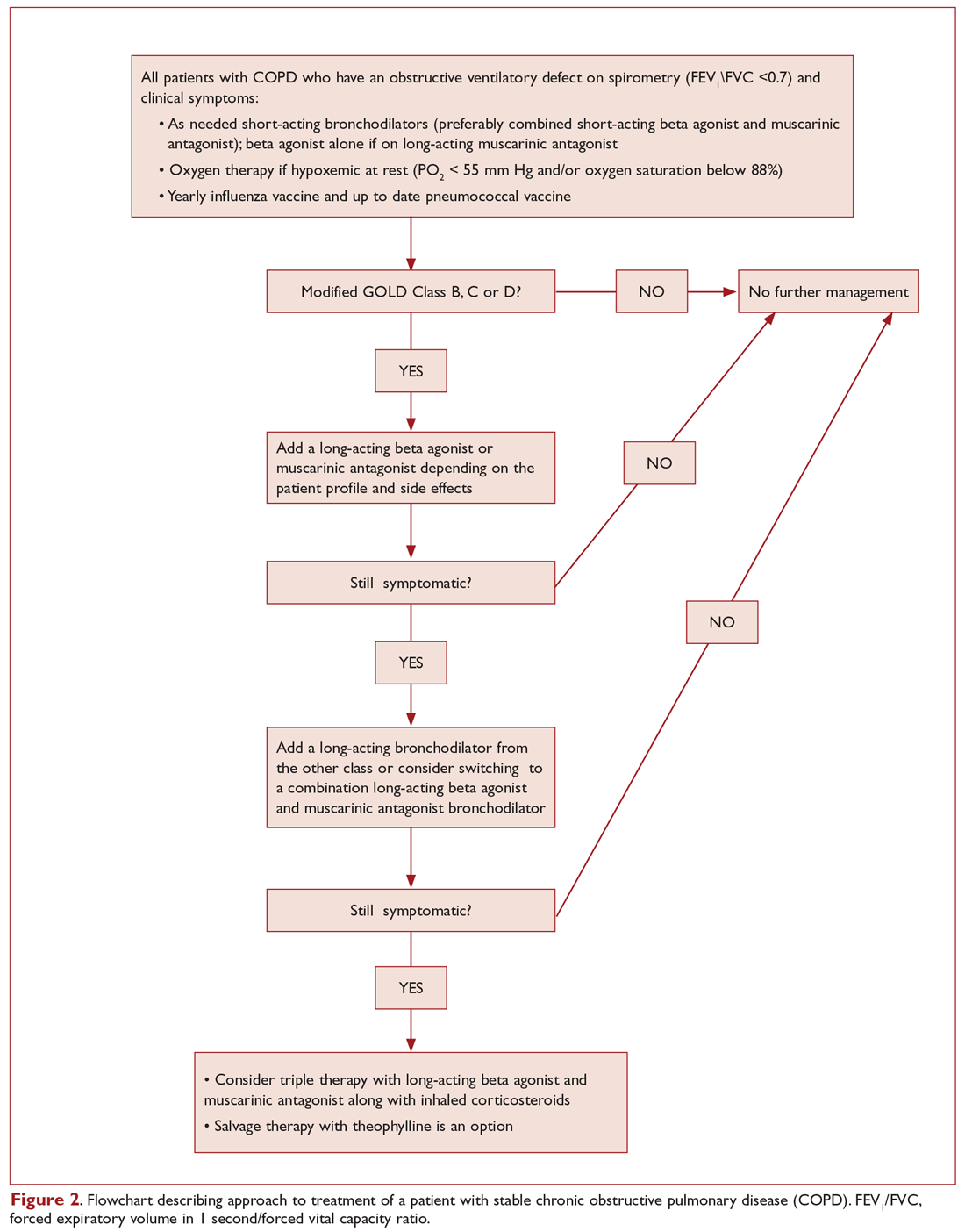
Figure 2 presents an approach to building a treatment plan for the patient with stable COPD.
Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms.7 Both short-acting beta-agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD9,10 and seem to have comparative efficacy when compared head-to-head in trials.11 However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with fewer exacerbations compared to albuterol alone.12 On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate.11-13 Inhaled short-acting beta-agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function.14
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta-agonists increased the risk of severe cardiac arrhythmias.15 Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia.16 Beta-agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side effects but achieve less than maximal bronchodilation.17 Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant, especially when combined with long-acting anticholinergic agents.18






