Diversion of Controlled Drugs in Hospitals: A Scoping Review of Contributors and Safeguards
Drug losses and theft from the healthcare system are accelerating; hospitals are pressured to implement safeguards to prevent drug diversion. Thus far, no reviews summarize all known risks and potential safeguards for hospital diversion. Past incidents of hospital drug diversion have impacted patient and staff safety, increased hospital costs, and resulted in infectious disease outbreaks. We searched MEDLINE, Embase, PsycINFO, CINAHL, Scopus, and Web of Science databases and the gray literature for articles published between January 2005 and June 2018. Articles were included if they focused on hospital settings and discussed either: (1) drug security or accounting practices (any drug) or (2) medication errors, healthcare worker substance use disorder, or incident reports (only with reference to controlled drugs). We included 312 articles and extracted four categories of data: (1) article characteristics (eg, author location), (2) article focus (eg, clinical areas discussed), (3) contributors to diversion (eg, factors enabling drug theft), and (4) diversion safeguards. Literature reveals a large number of contributors to drug diversion in all stages of the medication-use process. All health professions and clinical units are at risk. This review provides insights into known methods of diversion and the safeguards hospitals must consider to prevent them. Careful configuration of healthcare technologies and processes in the hospital environment can reduce the opportunity for diversion. These system-based strategies broaden the response to diversion beyond that of individual accountability. Further evidence is urgently needed to address the vulnerabilities outlined in this review and prevent harm.
© 2019 Society of Hospital Medicine
METHODS
Scoping Review
We followed Arksey and O’Malley’s six-step framework for scoping reviews,30 with the exception of the optional consultation phase (step 6). We addressed three questions (step 1): what clinical units, health professions, or stages of the medication-use process are commonly discussed; what are the identified contributors to diversion in hospitals; and what safeguards have been described for prevention or detection of diversion in hospitals? We then identified relevant studies (step 2) by searching records published from January 2005 to June 2018 in MEDLINE, Embase, PsycINFO, CINAHL, Scopus, and Web of Science; the gray literature was also searched (see supplementary material for search terms).
All study designs were considered, including quantitative and qualitative methods, such as experiments, chart reviews and audit reports, surveys, focus groups, outbreak investigations, and literature reviews. Records were included (step 3) if abstracts met the Boolean logic criteria outlined in Appendix 1. If no abstract was available, then the full-text article was assessed. Prior to abstract screening, four reviewers (including R.R.) independently screened batches of 50 abstracts at a time to iteratively assess interrater reliability (IRR). Disagreements were resolved by consensus and the eligibility criteria were refined until IRR was achieved (Fleiss kappa > 0.65). Once IRR was achieved, the reviewers applied the criteria independently. For each eligible abstract, the full text was retrieved and assigned to a reviewer for independent assessment of eligibility. The abstract was reviewed if the full-text article was not available. Only articles published in English were included.
Reviewers charted findings from the full-text records (steps 4 and 5) by using themes defined a priori, specifically literature characteristics (eg, authors, year of publication), characteristics related to study method (eg, article type), variables related to our research questions (eg, variations by clinical unit, health profession), contributors to diversion, and safeguards to detect or prevent diversion. Inductive additions or modifications to the themes were proposed during the full-text review (eg, reviewers added a theme “name of drugs diverted” to identify drugs frequently reported as diverted) and accepted by consensus among the reviewers.
RESULTS
Scoping Review
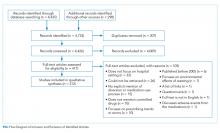
The literature search generated 4,733 records of which 307 were duplicates and 4,009 were excluded on the basis of the eligibility criteria. The reviewers achieved 100% interrater agreement on the fourth round of abstract screening. Upon full-text review, 312 articles were included for data abstraction (Figure).
Literature Characteristics
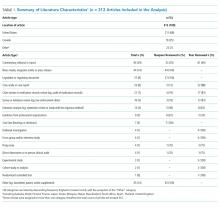
Table 1 summarizes the characteristics of the included literature. The articles were published in a mix of peer-reviewed (137, 44%) and nonpeer-reviewed (175, 56%) sources. Some peer-reviewed articles did not use research methods, and some nonpeer-reviewed articles used research methods (eg, doctoral theses). Therefore, Table 1 categorizes the articles by research method (if applicable) and by peer-review status. The articles primarily originated in the United States (211, 68%) followed by Canada (79, 25%) and other countries (22, 7%). Most articles were commentaries, editorials, reports or news media, rather than formal studies presenting original data.