Nephrotoxin-Related Acute Kidney Injury and Predicting High-Risk Medication Combinations in the Hospitalized Child
BACKGROUND: In the hospitalized patient, nephrotoxin exposure is one potentially modifiable risk factor for acute kidney injury (AKI). Clinical decision support based on nephrotoxin ordering was developed at our hospital to assist inpatient providers with the prevention or mitigation of nephrotoxin-related AKI. The initial decision support algorithm (Algorithm 1) was modified in order to align with a national AKI collaborative (Algorithm 2).
OBJECTIVE: Our first aim was to determine the impact of this alignment on the sensitivity and specificity of our nephrotoxin-related AKI detection system. Second, if the system efficacy was found to be suboptimal, we then sought to develop an improved model.
DESIGN: A retrospective cohort study in hospitalized patients between December 1, 2013 and November 30, 2015 (N = 14,779) was conducted.
INTERVENTIONS: With the goal of increasing nephrotoxin-related AKI detection sensitivity, a novel model based on the identification of combinations of high-risk medications was developed.
RESULTS: Application of the algorithms to our nephrotoxin use and AKI data resulted in sensitivities of 46.9% (Algorithm 1) and 43.3% (Algorithm 2, P = .22) and specificities of 73.6% and 89.3%, respectively (P < .001). Our novel AKI detection model was able to deliver a sensitivity of 74% and a specificity of 70%.
CONCLUSIONS: Modifications to our AKI detection system by adopting Algorithm 2, which included an expanded list of nephrotoxins and equally weighting each medication, did not improve our nephrotoxin-related AKI detection. It did improve our system’s specificity. Sensitivity increased by >50% when we applied a novel algorithm based on observed data with identification of key medication combinations.
© 2019 Society of Hospital Medicine
RESULTS
Sensitivity and Specificity
Of the patient encounters, 14,779 were eligible during the study period. The sensitivity of the system’s ability to identify nephrotoxin-related AKI decreased from 46.9% using Algorithm 1 to 43.3% using Algorithm 2, a change of 3.6% (P = .22). The specificity increased from 73.6% to 89.3%, a change of 15.7% (P < .001; Table 2).
Improvement of Our Nephrotoxin-Related AKI Detection System Using a Novel AKI Prediction Strategy
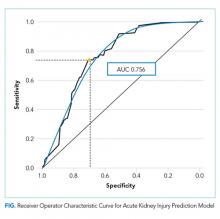
A total of 838 medication combinations were identified in our training set and the predicted AKI risk for every medication combination was determined. By comparing the predicted risk of AKI to the actual AKI occurrence, an ROC curve with an AUC of 0.756 (Figure) was generated. An increase in system sensitivity was prioritized when determining the optimal AKI risk at which the model would trigger an alert. Setting an alert threshold at a predicted AKI risk of >8%, our model performed with a sensitivity of 74% while decreasing the specificity to 70%.
Identification of High-Risk Nephrotoxic Medications and Medication Combinations
Approximately 200 medication combinations were associated with >8% AKI risk, our new AKI prediction model’s alert threshold. Medication combinations consisting of up to 11 concomitantly prescribed medications were present in our data set. However, many of these combinations were infrequently prescribed. Further analysis, conducted in order to increase the clinical relevance of our findings, identified 10 medications or medication combinations that were both associated with a predicted AKI risk of >8% and that were prescribed on average greater than twice a month (Table 3).
DISCUSSION
The nephrotoxin-related AKI detection system at SCH automatically places orders for serum creatinines on patients who have met criteria for concomitant nephrotoxin exposure. This has given us a robust database from which to develop our clinical decision-making tool. Both our original and updated systems were based on the absolute number of concomitant nephrotoxic medications prescribed.16 This is a reasonable approach given the complexity of building a surveillance system19 and resource limitations. However, a system based on observed rather than theoretical or in vitro data, adaptable to the institution and designed for ongoing refinement, would be more valuable.
The interest in AKI prediction tools continues to be high. Bedford et al. employed numerous variables and diagnostic codes to predict the development of AKI in adults during hospitalization. They were able to produce a prediction model with a reasonable fit (AUC 0.72) to identify patients at higher risk for AKI but were less successful in their attempts to predict progression to severe AKI.20 Hodgson et al. recently developed an adult AKI prediction score (AUC 0.65-0.72) also based on numerous clinical factors that was able to positively impact inpatient mortality.21 To our knowledge, our model is unique in that it focuses on nephrotoxins using a predicted AKI risk algorithm based on observed AKI rates of previously ordered medications/medication combinations (two to 11 medications). Having a decision tool targeting medications gives the clinician guidance that can be used to make a specific intervention rather than identifying a patient at risk due to a diagnosis code or other difficult to modify factors.
There are abundant case studies and reports using logistic regression models identifying specific medications associated with AKI. Our choice of methodology was based on our assessment that logistic regression models would be inadequate for the development of a real-time clinical decision-making tool for several reasons. Using logistic regression to explore every medication combination based on our medication list would be challenging as there are approximately 5.5 × 1010 potential medication combinations. Additionally, logistic regression ignores any potential interactions between the medications. This is an important point as medication interactions can be synergistic, neutral, or antagonist. Consequently, the outcome generated from a set of combined variables may be different from one generated from the sum of each variable taken independently. Logistic regression also does not account for the potential prescribing trends among providers as it assumes that all medications or medication combinations are equally available at the same time. However, in practice, depending on numerous factors, such as hospital culture (eg, the presence of clinical standard work pathways), local bacterial resistance patterns, or medication shortages; certain medication combinations may occur more frequently while others not at all. Finally, logistic regression cannot account for the possibility of a medication combination occurring; therefore, logistic regression may identify a combination strongly associated with AKI that is rarely prescribed.
We theorized that AKI detection would improve with the Algorithm 2 modifications, including the expanded nephrotoxin list, which accompanied alignment with the national pediatric AKI collaborative, NINJA. The finding that our surveillance sensitivity did not improve with this system update supported our subsequent objective to develop a novel nephrotoxin-related AKI decision tool or detection system using our EHR data to identify which specific medications and/or medication combinations were associated with a higher rate of AKI. However, it should be noted that two factors related to measurement bias introduce limitations to our sensitivity and specificity analyses. First, regarding the presence of the alert system, our system will order serum creatinines on patients when they have been exposed to nephrotoxins. Consequently, the proportion of patients with creatinines measured will increase in the nephrotoxin-exposed patients. Unexposed patients may have AKI that is not detected because creatinines may not be ordered. Therefore, there is the potential for a relative increase in AKI detection among nephrotoxin-exposed patients as compared with unexposed patients, which would then affect the measured sensitivity and specificity of the alert. Second, the automated alerts require a baseline creatinine in order to trigger therefore are unable to identify AKI among patients who do not have a baseline serum creatinine measurement.
Our new nephrotoxin-related AKI detection model performed best when an alert was triggered for those medications or medication combinations with a predicted AKI risk of >8%. Forty-six medication combinations consisting of exactly two medications were determined to have a predicted AKI risk of >8% therefore would trigger an alert in our new model system. These medication combinations would not have triggered an alert using either of the previous system algorithms as both algorithms are based on the presence of three or more concomitant nephrotoxic medications.
From the list of suspected nephrotoxins, we identified 11 unique medications in 10 different combinations with a predicted AKI risk of >8% that were prescribed frequently (at least twice a month on average; Table 3). Notably, six out of 10 medication combinations involved vancomycin. Piperacillin-tazobactam was also represented in several combinations. These findings support the concern that others have reported regarding these two medications particularly when prescribed together.22,23
Interestingly, enalapril was identified as a higher-risk medication both alone and in combination with another medication. We do not suspect that enalapril carries a higher risk than other angiotensin-converting enzyme (ACE) inhibitors to increase a patient’s serum creatinine. Rather, we suspect that in our hospitalized patients, this relatively short-acting ACE inhibitor is commonly used in several of our vulnerable populations such as in cardiac and bone marrow transplant patients.
The alert threshold of our model can be adjusted to increase either the sensitivity or the specificity of AKI detection. Our detection sensitivity increased by >1.5-fold with the alert trigger threshold set at a predicted AKI risk of >8%. As a screening tool, our alert limits could be set such that our sensitivity would be greater; however, balancing the potential for alert fatigue is important in determining the acceptance and, ultimately, the success of a working surveillance system.24
A patient’s overall risk of AKI is influenced by many factors such as the presence of underlying chronic comorbidities and the nature or severity of the acute illness as this may affect the patient’s intravascular volume status, systemic blood pressures, or drug metabolism. Our study is limited as we are a children’s hospital and our patients may have fewer comorbidities than seen in the adult population. One could argue that this permits a perspective not clouded by the confounders of chronic disease and allows for the effect of the medications prescribed to be more apparent. However, our study includes critically ill patients and patients who may have been hemodynamically unstable. This may explain why the NINJA algorithm did not improve the sensitivity of our AKI detection as the NINJA collaborative excludes critically ill patients.
Dose and dosing frequency of the prescribed medications could not be taken into account, which could explain the finding that nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, or ketorolac when used alone were associated with a low (<1%) rate of AKI despite being frequently prescribed. Additionally, as many providers are aware of the AKI risk of NSAIDs, these medications may have been used intermittently (as needed) or in select, perhaps healthier, patients or in patients that take these medications chronically who were admitted for reasons that did not alter their outpatient medication regimen.
Our study also reflects the prescribing habits of our institution and may not be directly applicable to nontertiary care hospitals or centers that do not have large cystic fibrosis, bone marrow, or solid organ transplant populations. Despite our study’s limitations, we feel that there are several findings that are relevant across centers and populations. Our data were derived from the systematic ordering of daily serum creatinines when a patient is at risk for nephrotoxin-related AKI. This is in step with the philosophy advocated by others that AKI identification can only occur if the providers are aware of this risk and are vigilant.25 In this vigilance, we also recognize that not all risks are of the same magnitude and may not deserve the same attention when resources are limited. Our identification of those medication combinations most associated with AKI at our institution has helped us narrow our focus and identify specific areas of potential education and intervention. The specific combinations identified may also be relevant to similar institutions serving similarly complex patients. Those with dissimilar populations could use this methodology to identify those medication combinations most relevant for their patient population and their prescriber’s habits. More studies of this type would be beneficial to the medical community as a whole as certain medication combinations may be found to be high risk regardless of the institution and the age or demographics of the populations they serve.