Things We Do for No Reason: Routine Echocardiography in Hemodynamically Stable Patients with Acute Pulmonary Embolism
© 2019 Society of Hospital Medicine
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
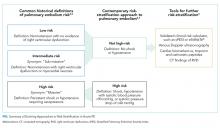
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11